Answered step by step
Verified Expert Solution
Question
1 Approved Answer
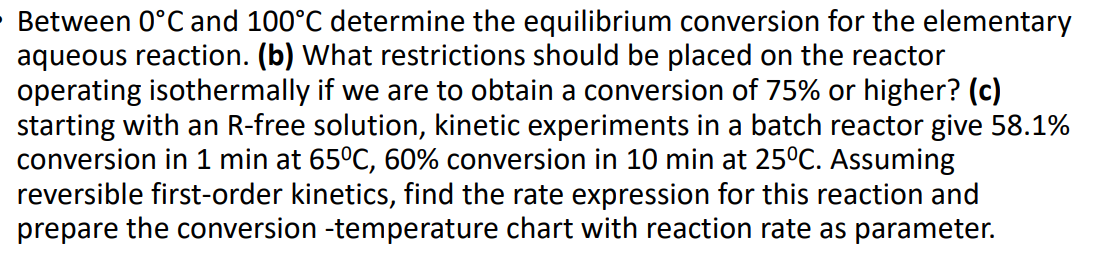
Between 0 C and 1 0 0 C determine the equilibrium conversion for the elementary aqueous reaction. ( b ) What restrictions should be placed
Between and determine the equilibrium conversion for the elementary
aqueous reaction. b What restrictions should be placed on the reactor
operating isothermally if we are to obtain a conversion of or higher? c
starting with an Rfree solution, kinetic experiments in a batch reactor give
conversion in min at conversion in min at Assuming
reversible firstorder kinetics, find the rate expression for this reaction and
prepare the conversion temperature chart with reaction rate as parameter.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


