Answered step by step
Verified Expert Solution
Question
1 Approved Answer
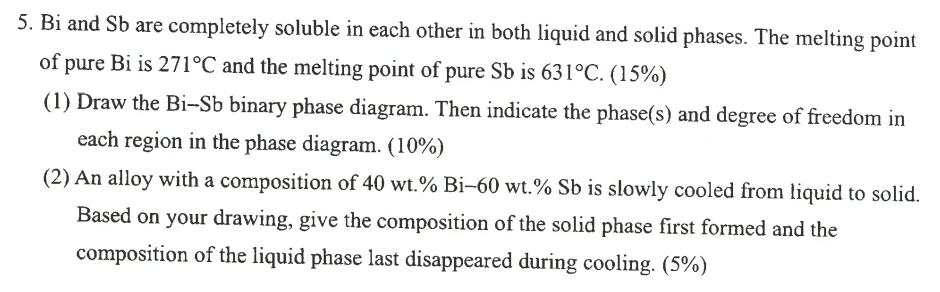
Bi and Sb are completely soluble in each other in both liquid and solid phases. The melting point of pure B i is 2 7
Bi and Sb are completely soluble in each other in both liquid and solid phases. The melting point
of pure is and the melting point of pure is
Draw the BiSb binary phase diagram. Then indicate the phases and degree of freedom in
each region in the phase diagram.
An alloy with a composition of is slowly cooled from liquid to solid.
Based on your drawing, give the composition of the solid phase first formed and the
composition of the liquid phase last disappeared during cooling.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


