Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Boltzmann famously defined the relation between the number of microstates W in an isolated system and the system's entropy by S = kB ln
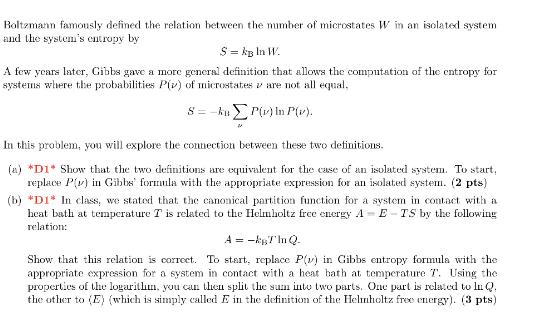
Boltzmann famously defined the relation between the number of microstates W in an isolated system and the system's entropy by S = kB ln W. A few years later, Gibbs gave a more general definition that allows the computation of the entropy for systems where the probabilities P() of microstates are not all equal. 8=-kuP(v) In P(v). 28 In this problem, you will explore the connection between these two definitions. (a) *D1* Show that the two definitions are equivalent for the case of an isolated system. To start, replace P() in Gibbs formula with the appropriate expression for an isolated system. (2 pts) (b) *DI* In class, we stated that the canonical partition function for a system in contact with a heat bath at temperature T is related to the Helmholtz free energy A-E-TS by the following relation: A = -T'In Q. Show that this relation is correct. To start, replace P() in Gibbs entropy formula with the appropriate expression for a system in contact with a heat bath at temperature T. Using the properties of the logarithm, you can then split the sum into two parts. One part is related to ln Q. the other to (E) (which is simply called E in the definition of the Helmholtz free energy). (3 pts)
Step by Step Solution
★★★★★
3.62 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


