Answered step by step
Verified Expert Solution
Question
1 Approved Answer
box answer A student wanted to measure the density, in g/ml, of an unknown metal alloy. They had multiple sized samples of this metal alloy
box answer 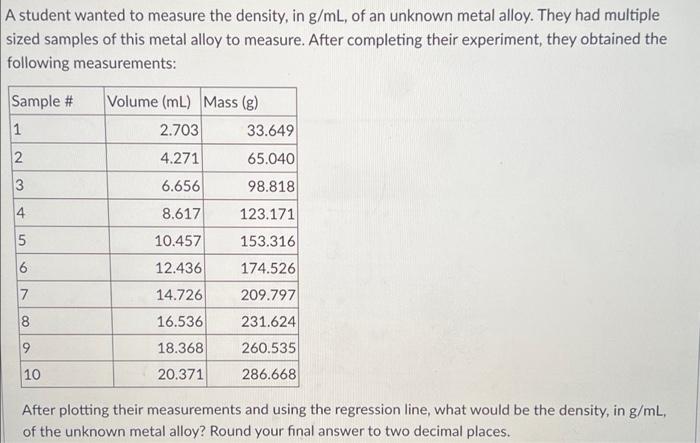
A student wanted to measure the density, in g/ml, of an unknown metal alloy. They had multiple sized samples of this metal alloy to measure. After completing their experiment, they obtained the following measurements: Sample # Volume (ml) Mass (g) 1 2.703 33.649 2 4.271 65.040 3 6.656 98.818 4 8.617 123.171 5 10.457 153.316 6 12.436 174.526 14.726 209.797 8 16.536 231.624 260.535 9 18.368 20.371 10 286.668 After plotting their measurements and using the regression line, what would be the density, in g/mL, of the unknown metal alloy? Round your final answer to two decimal places 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


