Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Bromination of an alkene by N-bromosuccinimide (NBS) in thepresence of light or peroxide is a radical reaction and produces anallylic bromide. For the following bromination
Bromination of an alkene by N-bromosuccinimide (NBS) in thepresence of light or peroxide is a radical reaction and produces anallylic bromide. For the following bromination of3-methylcyclopentene, select the allylic bromides from the set atthe right that would be products of the reaction.
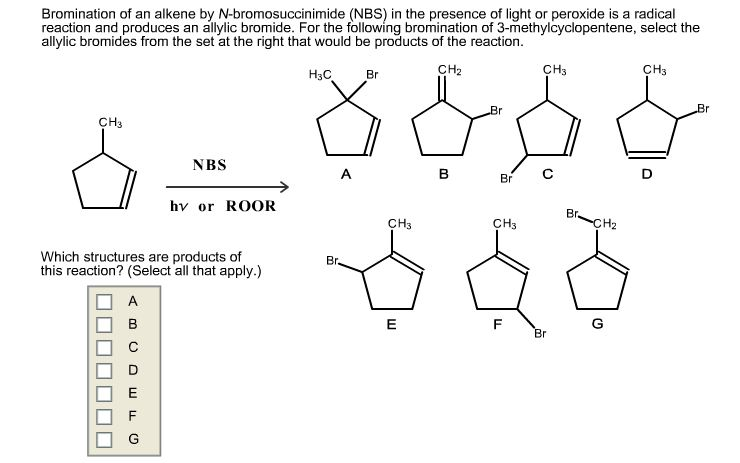
Bromination of an alkene by N-bromosuccinimide (NBS) in the presence of light or peroxide is a radical reaction and produces an allylic bromide. For the following bromination of 3-methylcyclopentene, select the allylic bromides from the set at the right that would be products of the reaction. CH3 A B Which structures are products of this reaction? (Select all that apply.) E NBS G hv or ROOR CH CH3 H3C Br Br B & S A B Br. CH3 E Br Br CH CH3 88 F Br (2 CH3 Br
Step by Step Solution
There are 3 Steps involved in it
Step: 1
I aec is not ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


