Answered step by step
Verified Expert Solution
Question
1 Approved Answer
C Get Homework Help With Chego X C In A Voltaic Cell, A Strip Of Galliu Alef Telegram + moe.alefed.com/21646423517671#/progress/interim/9fb7d6c5-5da1-4615-84a0-42125498a9ca/classid/Sebfe906-39e3-480d-85bc-48c3a399ac71/grou... GE : X 47. Checkpoint
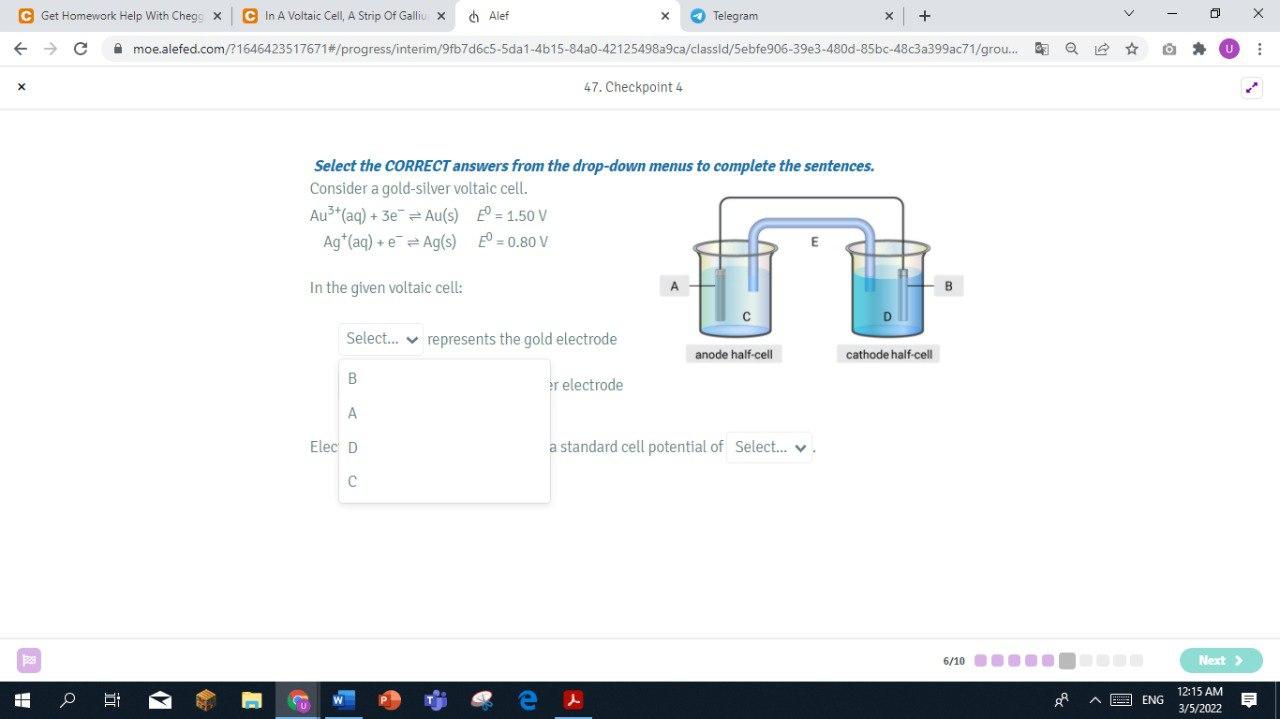
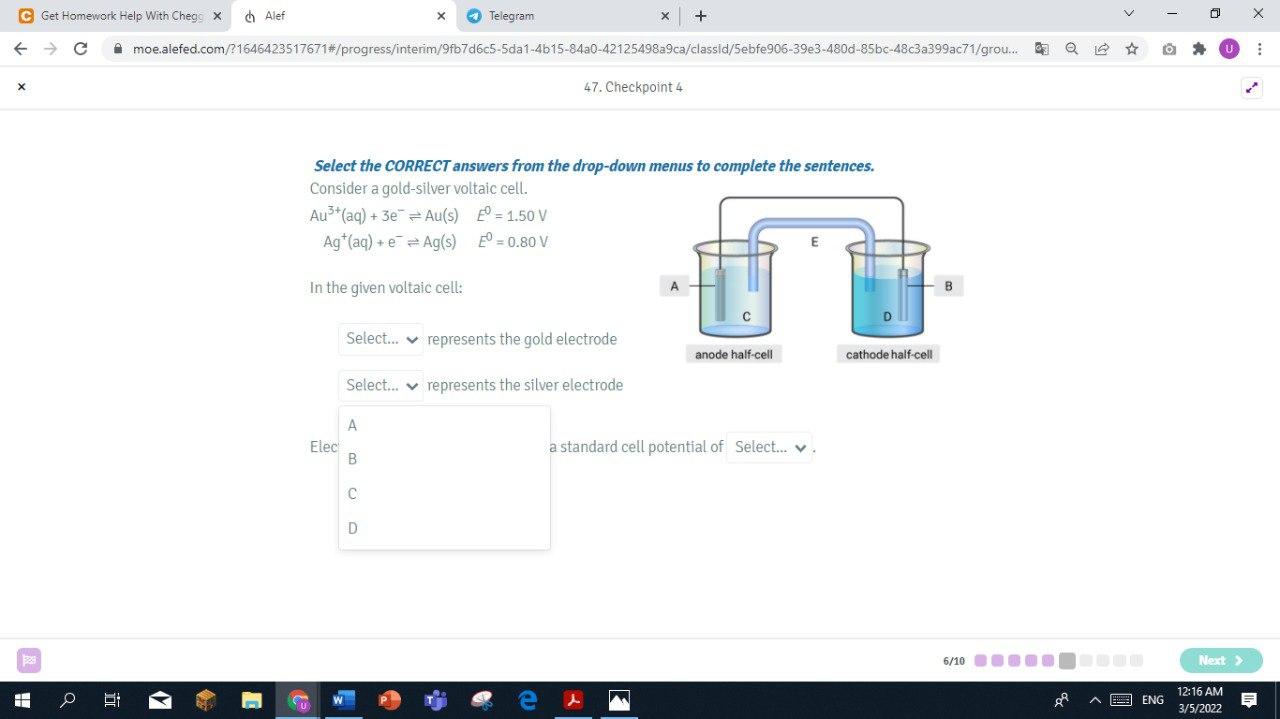
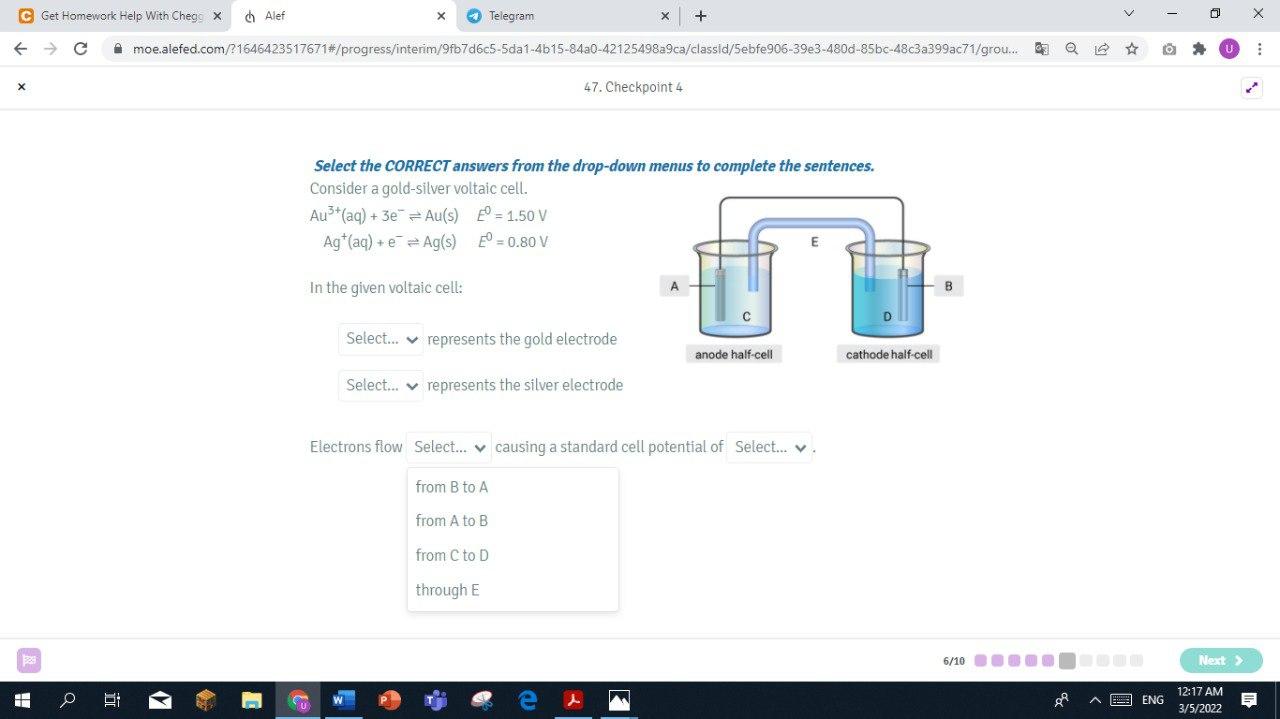
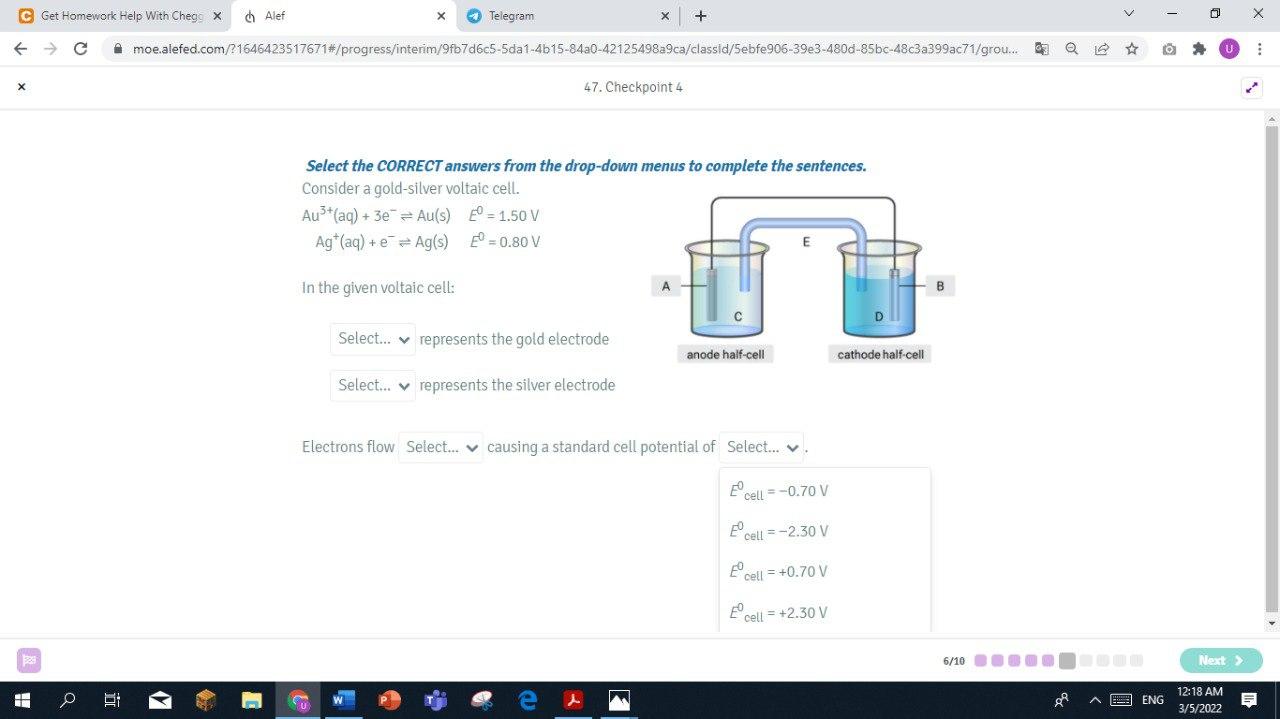
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


