Question
a) How many moles of CaBr2 would be consumed when reacted with 53.0 ml of a 2.00 M H2S04 solution? b) If you consume
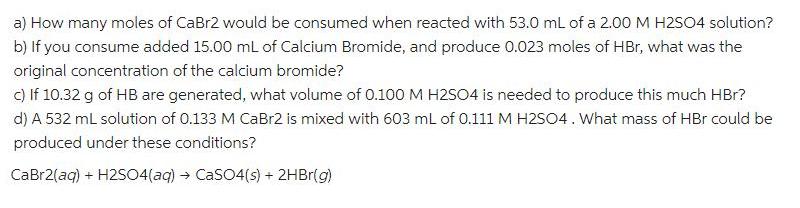
a) How many moles of CaBr2 would be consumed when reacted with 53.0 ml of a 2.00 M H2S04 solution? b) If you consume added 15.00 mL of Calcium Bromide, and produce 0.023 moles of HBr, what was the original concentration of the calcium bromide? c) If 10.32 g of HB are generated, what volume of 0.100 M H2504 is needed to produce this much HBr? d) A 532 mL solution of 0.133 M CAB12 is mixed with 603 mL of 0.111 M H2SO4. What mass of HBr could be produced under these conditions? CaBr2(ag) + H2S04(aq) CaSO4(s) + 2HBr(g)
Step by Step Solution
3.54 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
a mol of H2SO4 MV 2 53103 0106 mol of H2S04 ratio is 11 so mol of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



