Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate and plot the concentration of potassium, aluminium and sulphate ions as a function of distance from the surface. The required units are mM
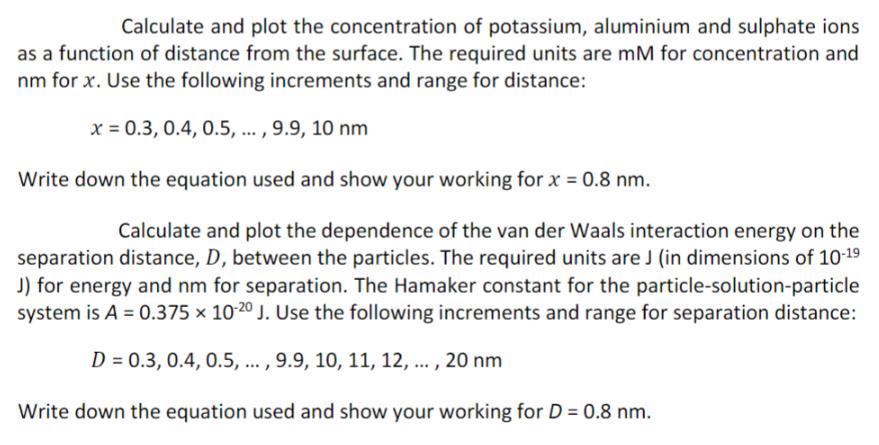
Calculate and plot the concentration of potassium, aluminium and sulphate ions as a function of distance from the surface. The required units are mM for concentration and nm for x. Use the following increments and range for distance: x = 0.3, 0.4, 0.5, ..., 9.9, 10 nm Write down the equation used and show your working for x = 0.8 nm. Calculate and plot the dependence of the van der Waals interaction energy on the separation distance, D, between the particles. The required units are J (in dimensions of 10-19 J) for energy and nm for separation. The Hamaker constant for the particle-solution-particle system is A = 0.375 x 10-20 J. Use the following increments and range for separation distance: D = 0.3, 0.4, 0.5, ..., 9.9, 10, 11, 12,..., 20 nm Write down the equation used and show your working for D = 0.8 nm.
Step by Step Solution
★★★★★
3.40 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


