Answered step by step
Verified Expert Solution
Question
1 Approved Answer
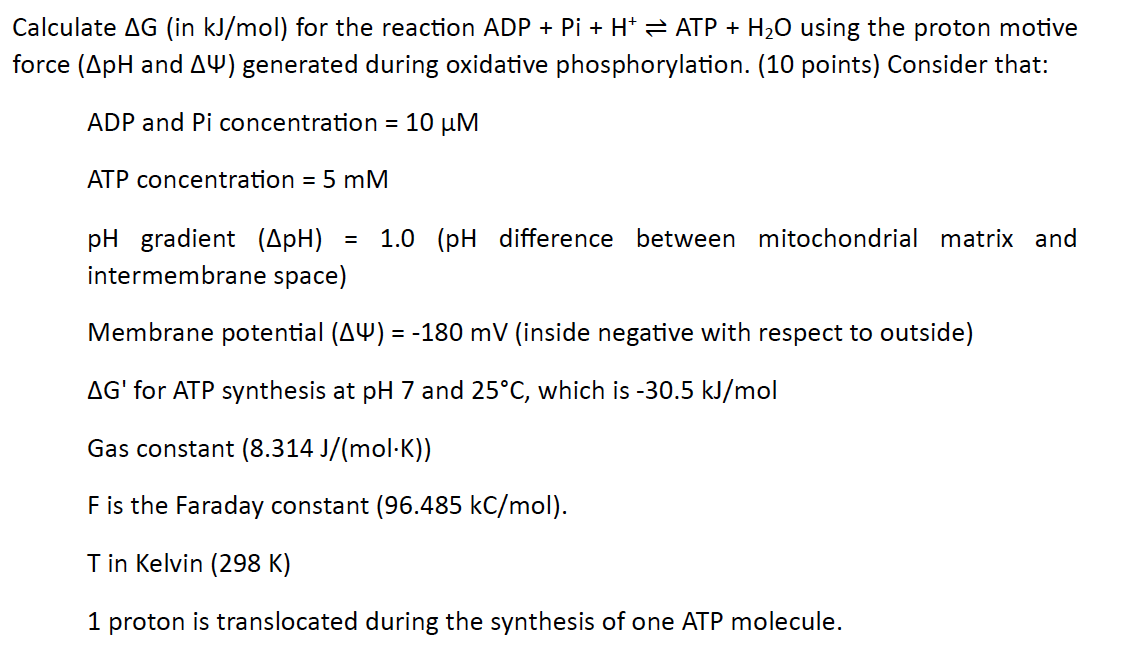
Calculate G ( in k J m o l ) for the reaction ADP + + H + A T P + H 2 O
Calculate in for the reaction ADP using the proton motive
force and generated during oxidative phosphorylation. points Consider that:
ADP and concentration
ATP concentration
gradient difference between mitochondrial matrix and
intermembrane space
Membrane potential inside negative with respect to outside
for ATP synthesis at and which is
Gas constant
is the Faraday constant
T in Kelvin K
proton is translocated during the synthesis of one ATP molecule.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


