Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate Molarity 2.5x10^-3M potassium ferricyanide solution Mass= .2055 (or .21) Calculate the Molarity of potassium ferricyanide what other data is needed? Hoping this helps with
Calculate Molarity 

2.5x10^-3M potassium ferricyanide solution
Calculate the Molarity of potassium ferricyanide
what other data is needed?
Hoping this helps with data 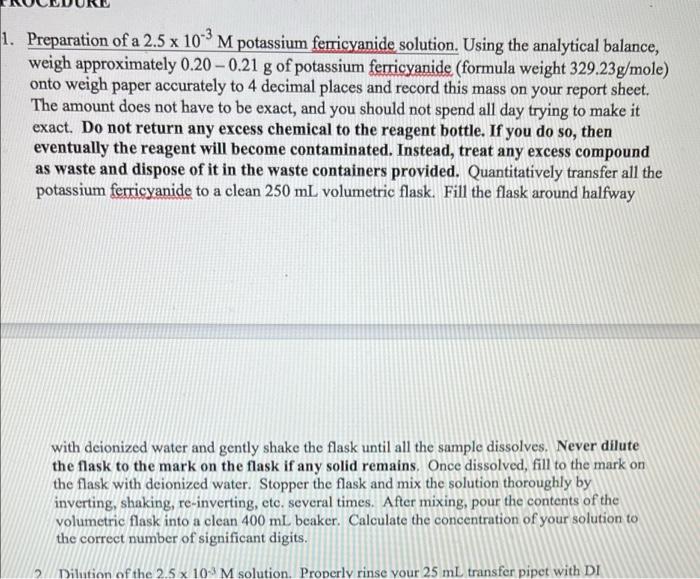
1. Preparation of 2.5 x 10-'M potassium ferricyanide solution Mass of potassium ferricyanide .2055 Molarity of potassium ferricyanide Show calculation for molarity of potassium ferricyanide; X 1. Preparation of a 2.5 x 10-2 M potassium ferricyanide solution. Using the analytical balance, weigh approximately 0.20 -0.21 g of potassium ferricyanide (formula weight 329.23g/mole) onto weigh paper accurately to 4 decimal places and record this mass on your report sheet. The amount does not have to be exact, and you should not spend all day trying to make it exact. Do not return any excess chemical to the reagent bottle. If you do so, then eventually the reagent will become contaminated. Instead, treat any excess compound as waste and dispose of it in the waste containers provided. Quantitatively transfer all the potassium ferricyanide to a clean 250 mL volumetric flask. Fill the flask around halfway a with deionized water and gently shake the flask until all the sample dissolves. Never dilute the flask to the mark on the flask if any solid remains. Once dissolved, fill to the mark on the flask with deionized water. Stopper the flask and mix the solution thoroughly by inverting, shaking, re-inverting, etc. several times. After mixing, pour the contents of the volumetric flask into a clean 400 mL beaker. Calculate the concentration of your solution to the correct number of significant digits. 2 Dilution of the 2.5 x 10-4 M solution. Properly rinse your 25 ml transfer pipet with DI 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


