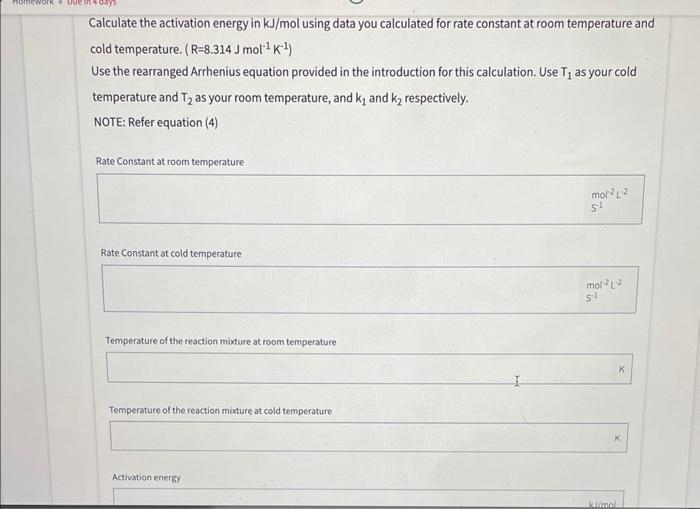
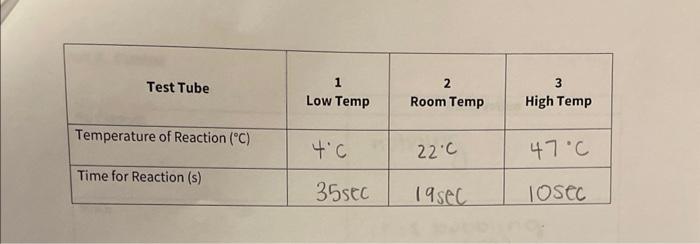
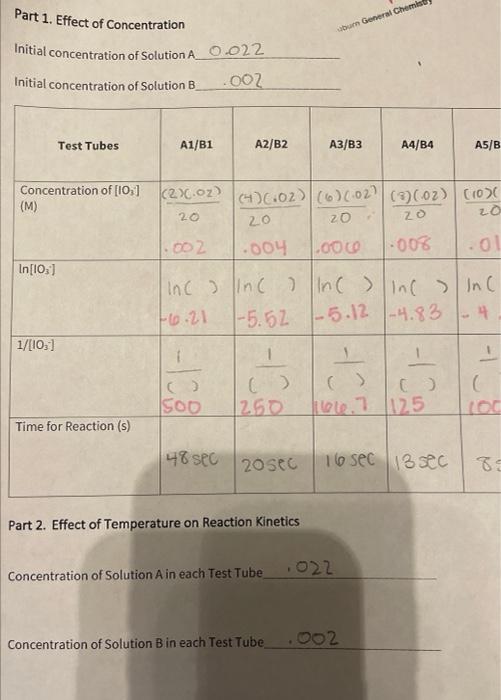
Calculate the activation energy in kJ/mol using data you calculated for rate constant at room temperature and cold temperature. (R=8.314Jmol1K1) Use the rearranged Arrhenius equation provided in the introduction for this calculation. Use T1 as your cold temperature and T2 as your room temperature, and k1 and k2 respectively. NOTE: Refer equation (4) Rate Constant at room temperature : Rate Constant at cold temperature Temperature of the reaction mixture at room temperature Temperature of the reaction mixture at cold temperature \begin{tabular}{|c|c|c|c|} \hline \multicolumn{1}{|c|}{ Test Tube } & 1 & 2 \\ Low Temp & Room Temp & 3HighTemp \\ \hline Temperature of Reaction (C) & 4C & 22C & 47C \\ \hline Time for Reaction (s) & 35seC & 19seC & 10SeC \\ \hline \end{tabular} part 3) hature of The reactant - Do one test tube at a time lable 1 - 5 place 1mv of into eacn test tube as follows 13MH2SO426MHCl36MHNO342MH2HO456MCH3COOH - Obtain a bong Mg ribbon and polisned until it shines. Cut into lom pieces. (you need s puces) - check weignt + make sune they have similar masses - place lcm of riboon into first test tube t Quickly start watcht When mg is compuetely reacted stop watch t necora - reaction will convert riboon to coorosponding saltt liberate hydrogen gas. - nepeat same procedure for other a test tubes. - list in decreasing order of neactiontime Part 1. Effect of Concentration Initial concentration of Solution A 0.022 Initial concentration of Solution B.OO _ Part 2. Effect of Temperature on Reaction Kinetics Concentration of Solution A in each Test Tube_, , 022 Concentration of Solution B in each Test Tube . 002 Calculate the activation energy in kJ/mol using data you calculated for rate constant at room temperature and cold temperature. (R=8.314Jmol1K1) Use the rearranged Arrhenius equation provided in the introduction for this calculation. Use T1 as your cold temperature and T2 as your room temperature, and k1 and k2 respectively. NOTE: Refer equation (4) Rate Constant at room temperature : Rate Constant at cold temperature Temperature of the reaction mixture at room temperature Temperature of the reaction mixture at cold temperature \begin{tabular}{|c|c|c|c|} \hline \multicolumn{1}{|c|}{ Test Tube } & 1 & 2 \\ Low Temp & Room Temp & 3HighTemp \\ \hline Temperature of Reaction (C) & 4C & 22C & 47C \\ \hline Time for Reaction (s) & 35seC & 19seC & 10SeC \\ \hline \end{tabular} part 3) hature of The reactant - Do one test tube at a time lable 1 - 5 place 1mv of into eacn test tube as follows 13MH2SO426MHCl36MHNO342MH2HO456MCH3COOH - Obtain a bong Mg ribbon and polisned until it shines. Cut into lom pieces. (you need s puces) - check weignt + make sune they have similar masses - place lcm of riboon into first test tube t Quickly start watcht When mg is compuetely reacted stop watch t necora - reaction will convert riboon to coorosponding saltt liberate hydrogen gas. - nepeat same procedure for other a test tubes. - list in decreasing order of neactiontime Part 1. Effect of Concentration Initial concentration of Solution A 0.022 Initial concentration of Solution B.OO _ Part 2. Effect of Temperature on Reaction Kinetics Concentration of Solution A in each Test Tube_, , 022 Concentration of Solution B in each Test Tube . 002