Question
Calculate the amount of heat required to convert 1.00 kg of ice at -10C into steam at 100C at normal pressure. Specific heat capacity
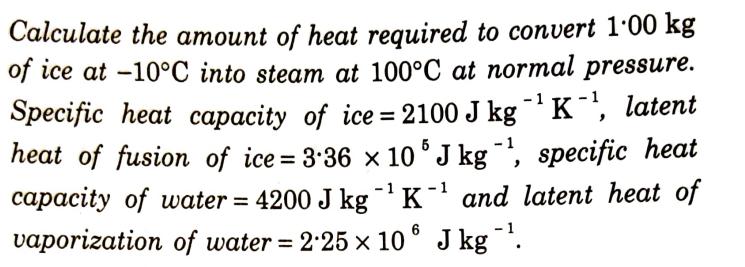
Calculate the amount of heat required to convert 1.00 kg of ice at -10C into steam at 100C at normal pressure. Specific heat capacity of ice=2100 J kg K, latent heat of fusion of ice = 3.36 x 105 J kg-, specific heat capacity of water = 4200 J kgK and latent heat of vaporization of water = 2.25 x 10 J kg .
Step by Step Solution
3.38 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Physics Coursebook
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
3rd Edition
1108859038, 978-1108859035
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



