Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the average atomic mass of the following mixture of elements using the Chart of the Nuclides. Element Percentage Fe-54 6.0% Fe-56 90.0% Fe-57


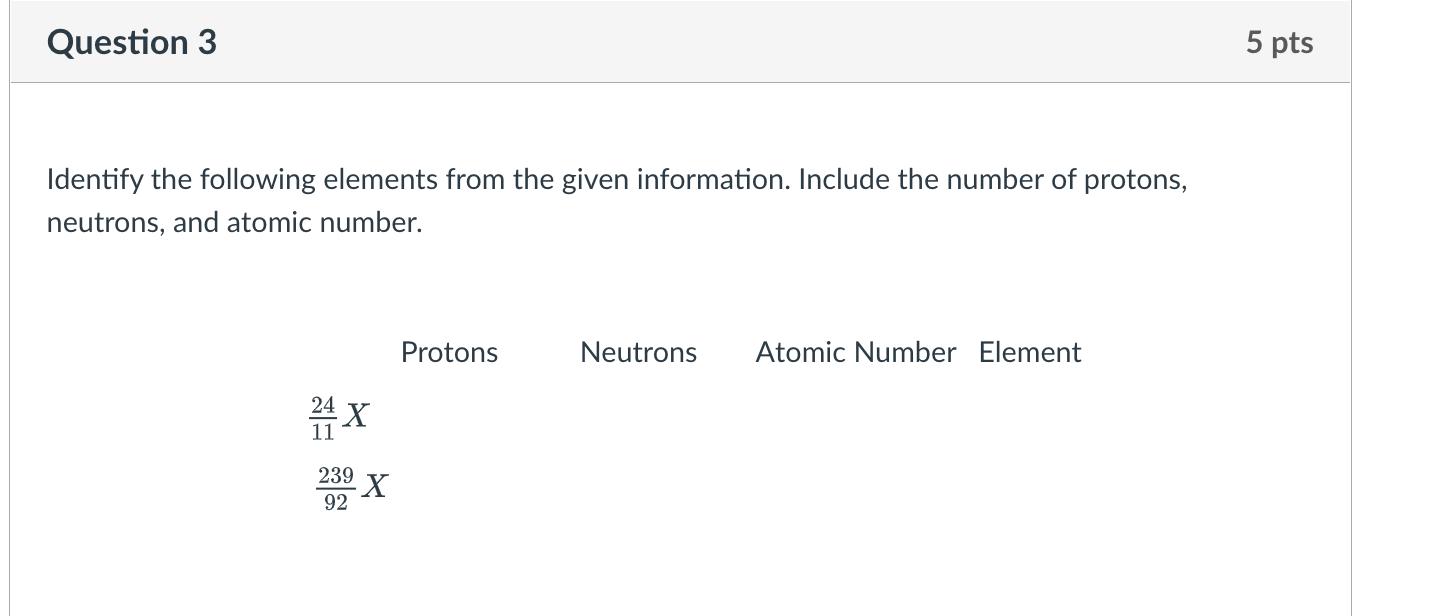
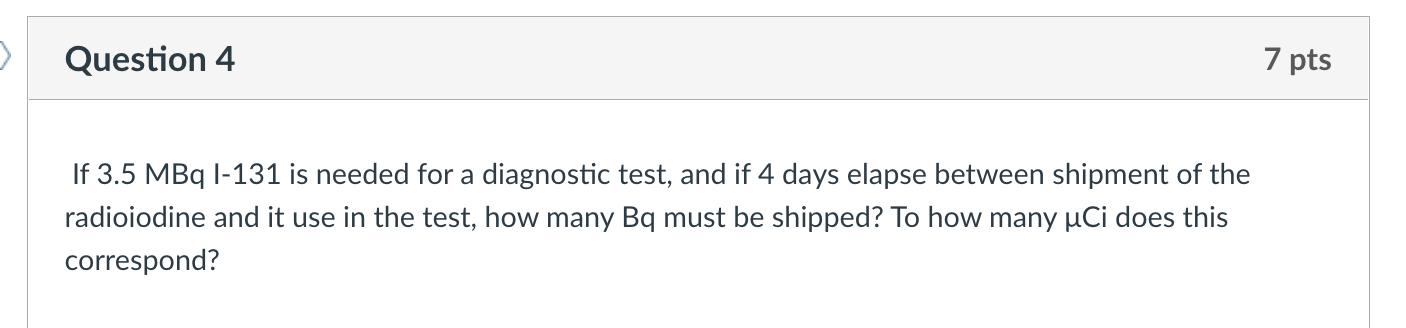
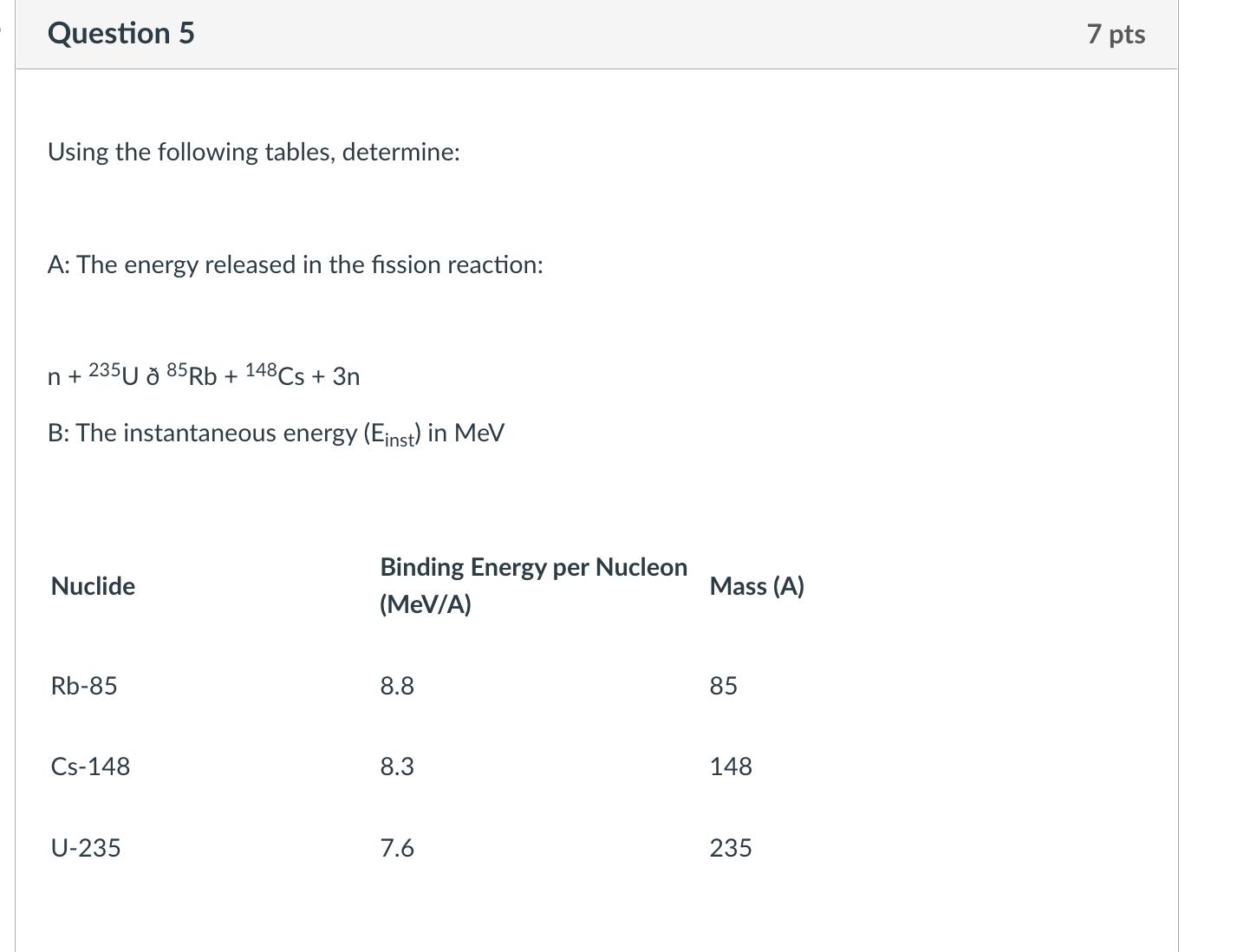
Calculate the average atomic mass of the following mixture of elements using the Chart of the Nuclides. Element Percentage Fe-54 6.0% Fe-56 90.0% Fe-57 3.0% Fe-58 1.0% Question 2 Calculate the energy equivalent of mass defect of a U-234 atom. 7 pts Question 3 Identify the following elements from the given information. Include the number of protons, neutrons, and atomic number. 239 X Protons Neutrons Atomic Number Element 5 pts Question 4 If 3.5 MBq I-131 is needed for a diagnostic test, and if 4 days elapse between shipment of the radioiodine and it use in the test, how many Bq must be shipped? To how many Ci does this correspond? 7 pts Question 5 Using the following tables, determine: A: The energy released in the fission reaction: n+235U 85 Rb + 148 Cs + 3n B: The instantaneous energy (Einst) in MeV Nuclide Binding Energy per Nucleon (MeV/A) Mass (A) Rb-85 8.8 85 CS-148 8.3 148 U-235 7.6 235 7 pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


