Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Acid Ionization Constants at 25C Name of Acid Ionization Equation K a Sulfuric acid H 2 SO 4 H + + HSO 4
| Name of Acid | Ionization Equation | Ka |
|---|---|---|
| Sulfuric acid | H2SO4 ⇌ H+ + HSO4− HSO4− ⇌ H+ + SO42− | very large 1.3 × 10−2 |
| Oxalic acid | H2C2O4 ⇌ H+ + HC2O4− HC2O4− ⇌ H+ + C2O42− | 6.5 × 10−2 6.1 × 10−5 |
| Phosphoric acid | H3PO4 ⇌ H+ + H2PO4− H2PO4− ⇌ H+ + HPO42− HPO42− ⇌ H+ + PO43− | 7.5 × 10−3 6.2 × 10−8 4.8 × 10−13 |
| Hydrofluoric acid | HF ⇌ H+ + F− | 7.1 × 10−4 |
| Nitrous acid | HNO2 ⇌ H+ + NO2− | 4.5 × 10−4 |
| Benzoic acid | C6H5COOH ⇌ H+ + C6H5COO− | 6.5 × 10−5 |
| Acetic acid | CH3COOH ⇌ H+ + CH3COO− | 1.8 × 10−5 |
| Carbonic acid | H2CO3 ⇌ H+ + HCO3− HCO3− ⇌ H+ + CO32− | 4.2 × 10−7 4.8 × 10−11 |
| Hydrocyanic acid | HCN ⇌ H+ + CN− | 4.9 × 10−10 |
| Name of Base | Ionization Equation | Kb |
|---|---|---|
| Methylamine | CH3NH2 + H2O ⇌ CH3NH3+ + OH− | 5.6 × 10−4 |
| Ammonia | NH3 + H2O ⇌ NH4+ + OH− | 1.8 × 10−5 |
| Pyridine | C5H5N + H2O ⇌ C5H5NH+ + OH− | 1.7 × 10−9 |
| Acetate ion | CH3COO− + H2O ⇌ CH3COOH + OH− | 5.6 × 10−10 |
| Fluoride ion | F− + H2O ⇌ HF + OH− | 1.4 × 10−11 |
| Urea | H2NCONH2 + H2O ⇌ H2CONH3+ + OH− | 1.5 × 10−14 |
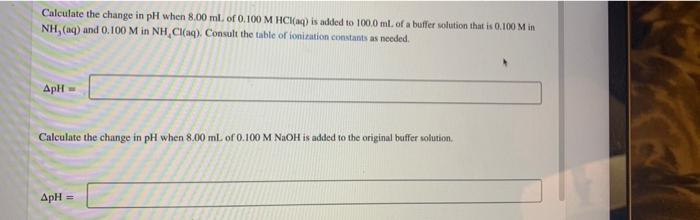
Calculate the change in pH when 8.00 ml. of 0.100 M HCI(aq) is added to 100.0 ml. of a buffer solution that is 0.100 M in NH, (aq) and 0.100 M in NH, CI(aq). Consult the table of ionization constants as needed. ApH Calculate the change in pH when 8.00 ml of 0.100 M NAOH is added to the original buffer solution. ApH =
Step by Step Solution
★★★★★
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
63617894963ff_235774.pdf
180 KBs PDF File
63617894963ff_235774.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


