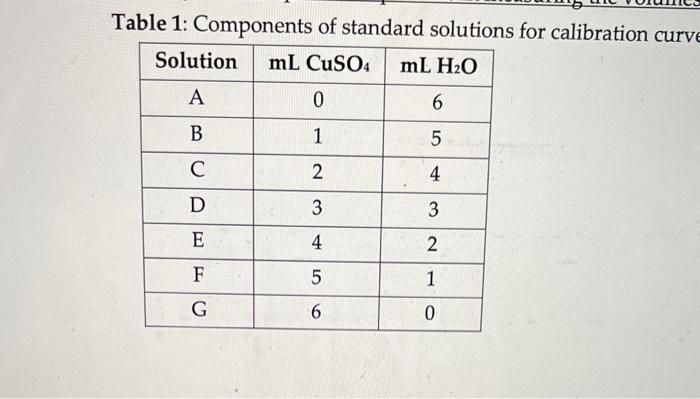
Calculate the concentrations of the six solutions listed in Table 1 of the Procedure. Copy these values onto your Cover Page for your use during the lab. Report the concentration of solution F below. Round it to two sig figs, and include the unit. Use M for your units. An answer could be 0.23M Table 1: Components of standard solutions for calibration curr Part I. Chemical Transformations Involving Copper Starting with copper (II) nitrate, you will successively transform the compound into the following forms: Copper (II) hydroxide Copper (II) oxide Copper (II) sulfate Procedure 1. In a 250mL beaker, dissolve about 2.0 grams of copper (II) nitrate in about 100mL of distilled water. Record the actual mass used to 0.1mg. 2. Slowly add about 10.0mL of 6M sodium hydroxide to the beaker of copper (II) nitrate solution. Check the pH of the solution with a piece of litmus paper. If the solution is not basic, add a few more mL of sodium hydroxide and check again. The compound formed here is copper (II) hydroxide. 3. Heat the solution on a hot plate to a gentle boil; avoid heating it too strongly such that it splatters. Do not turn the hot plate above " 200C" (or medium heat). Use a stir bar on medium speed. 4. Allow the mixture to digest at 100C for about 1015 minutes. The new precipitate should settle to the bottom of the beaker. After the digestion process, allow the solution to cool down to about room temperature. 5. Decant as much water from the beaker as possible without pouring out any solid. Discard the liquid in the waste container. 6. Filter the solid using gravity filtration. This can be done using a fluted piece of filter paper and a funnel. Use an Erlenmeyer flask to catch the liquid from the filtration process. 7. Use cold distilled water to transfer any residual solid through the funnel. The filtered liquid can be discarded. The solid on the filter paper is copper (II) oxide. 8. Pour about 25.0mL of 3.0M sulfuric acid slowly over the solid on the filter paper, collecting the liquid below in a clean dry Erlenmeyer flask. The liquid collected contains copper (II) sulfate. 9. Check the filter paper for any remaining solid. a. If some solid remains, place the solid and filter paper in a small beaker and wash it with an additional 5mL of 3.0M sulfuric acid. Combine this wash with the liquid collected already. b. If all of the solid dissolved, wash the filter paper a few times with small amounts of distilled water. The wash liquid should be collected in the same Erlenmeyer flask as above. 10. Keep the solution in the flask for later use in Part III. Discard the filter paper in the solid waste (not the trash). 11. Answer th 4 questions for Part I of the In-Class Assignment. Part II. Generating a Calibration Curve for Copper (II) Sulfate You will now generate a calibration curve by measuring the absorbance of a series of copper (II) sulfate solutions of known concentration that you will prepare. This will be used in Part III when you determine the concentration of your product from Part I. Procedure 1. You will prepare the following seven solutions. Label seven clean small test tubes appropriately. It is important that you are as precise as possible in measuring the volumes below. Table 1: Components of standard solutions for calibration curve. Calculate the concentrations of the six solutions listed in Table 1 of the Procedure. Copy these values onto your Cover Page for your use during the lab. Report the concentration of solution F below. Round it to two sig figs, and include the unit. Use M for your units. An answer could be 0.23M Table 1: Components of standard solutions for calibration curr Part I. Chemical Transformations Involving Copper Starting with copper (II) nitrate, you will successively transform the compound into the following forms: Copper (II) hydroxide Copper (II) oxide Copper (II) sulfate Procedure 1. In a 250mL beaker, dissolve about 2.0 grams of copper (II) nitrate in about 100mL of distilled water. Record the actual mass used to 0.1mg. 2. Slowly add about 10.0mL of 6M sodium hydroxide to the beaker of copper (II) nitrate solution. Check the pH of the solution with a piece of litmus paper. If the solution is not basic, add a few more mL of sodium hydroxide and check again. The compound formed here is copper (II) hydroxide. 3. Heat the solution on a hot plate to a gentle boil; avoid heating it too strongly such that it splatters. Do not turn the hot plate above " 200C" (or medium heat). Use a stir bar on medium speed. 4. Allow the mixture to digest at 100C for about 1015 minutes. The new precipitate should settle to the bottom of the beaker. After the digestion process, allow the solution to cool down to about room temperature. 5. Decant as much water from the beaker as possible without pouring out any solid. Discard the liquid in the waste container. 6. Filter the solid using gravity filtration. This can be done using a fluted piece of filter paper and a funnel. Use an Erlenmeyer flask to catch the liquid from the filtration process. 7. Use cold distilled water to transfer any residual solid through the funnel. The filtered liquid can be discarded. The solid on the filter paper is copper (II) oxide. 8. Pour about 25.0mL of 3.0M sulfuric acid slowly over the solid on the filter paper, collecting the liquid below in a clean dry Erlenmeyer flask. The liquid collected contains copper (II) sulfate. 9. Check the filter paper for any remaining solid. a. If some solid remains, place the solid and filter paper in a small beaker and wash it with an additional 5mL of 3.0M sulfuric acid. Combine this wash with the liquid collected already. b. If all of the solid dissolved, wash the filter paper a few times with small amounts of distilled water. The wash liquid should be collected in the same Erlenmeyer flask as above. 10. Keep the solution in the flask for later use in Part III. Discard the filter paper in the solid waste (not the trash). 11. Answer th 4 questions for Part I of the In-Class Assignment. Part II. Generating a Calibration Curve for Copper (II) Sulfate You will now generate a calibration curve by measuring the absorbance of a series of copper (II) sulfate solutions of known concentration that you will prepare. This will be used in Part III when you determine the concentration of your product from Part I. Procedure 1. You will prepare the following seven solutions. Label seven clean small test tubes appropriately. It is important that you are as precise as possible in measuring the volumes below. Table 1: Components of standard solutions for calibration curve