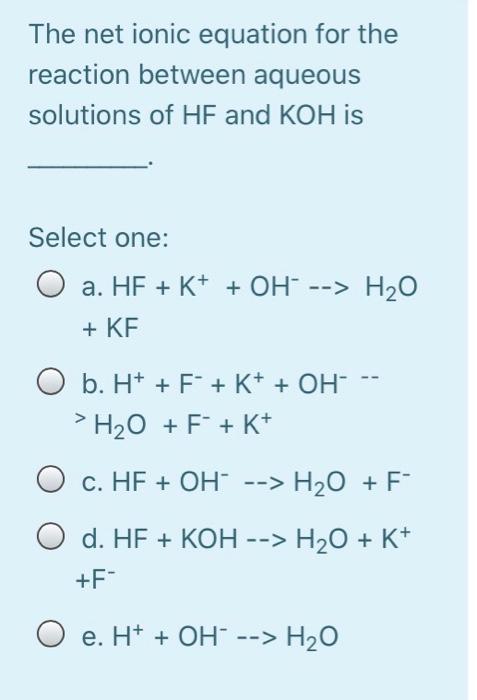
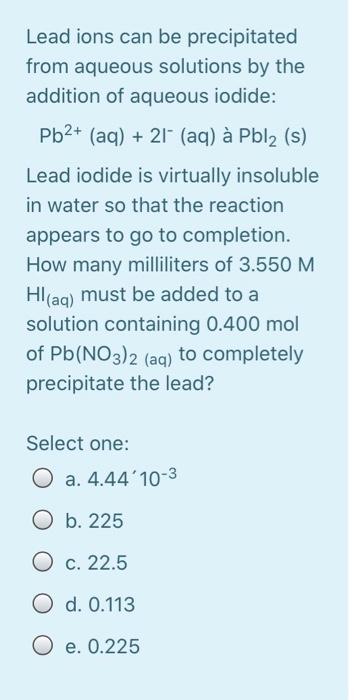
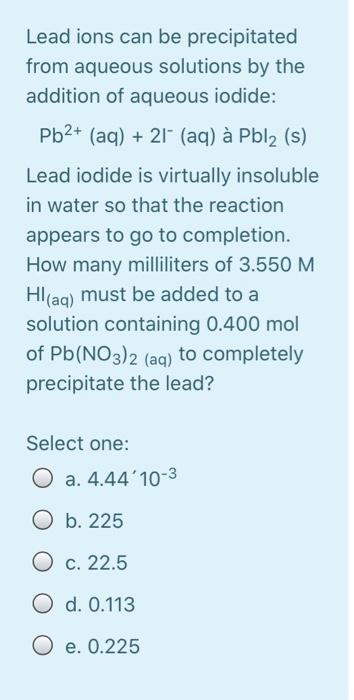
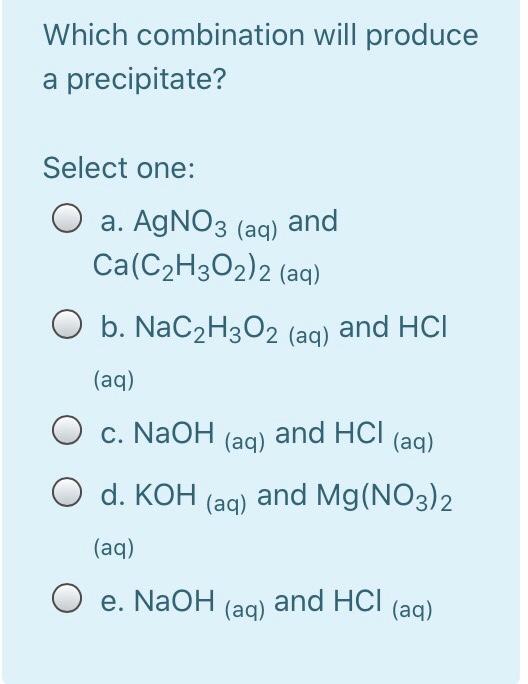
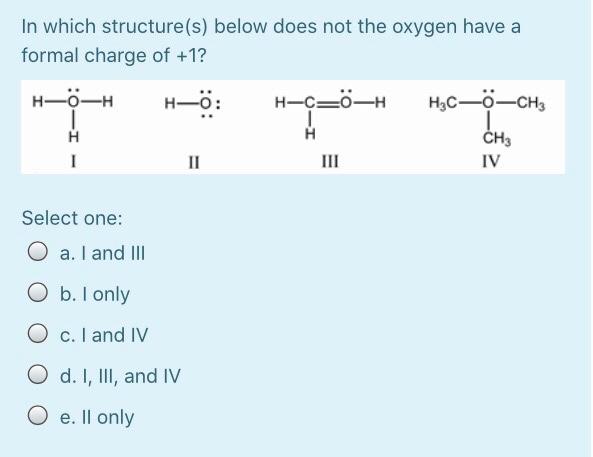
Calculate the energy required to excite a hydrogen atom by causing an electronic transition from the energy level with n = 1 to the level with n = 4. Select one: O a. 2.254 x 10-18 J O b. 2.192 x 105 J O c. 2.043 x 10-18 J O d. 3.275 x 10-17 O e. 2.017 x 10-29 J The net ionic equation for the reaction between aqueous solutions of HF and KOH is Select one: O a. HF + K+ + OH --> H2O + KF O b. H+ + F + K+ + OH- H2O + F + K+ O c. HF + OH --> H2O + F" O d. HF + KOH --> H2O + K+ +F- O e. H+ + OH --> H2O A strong electrolyte is one that completely in solution. Select one: a. decomposes b. disappears c. reacts d. ionizes O e. All are correct The hybridizations of iodine in IFs and IF3 are and respectively. Select one: a. sp3d?, sp3d b. sp3, sp3d C. sp3d2, sp3d O d. sp3d, sp3d2 O e. sp3d, sp3 What bond angle is associated with a TetrachloroCarbon molecule? Select one: O a. 45 b. 180 c. 90 O d. 109.5 O e. 120 Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: Pb2+ (aq) + 21+ (aq) Pbl2 (s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion. How many milliliters of 3.550 M Hl(aq) must be added to a solution containing 0.400 mol of Pb(NO3)2 (aq) to completely precipitate the lead? Select one: O a. 4.44'10-3 O b. 225 O c. 22.5 O d. 0.113 O e. 0.225 Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: Pb2+ (aq) + 21+ (aq) Pbl2 (s) Lead iodide is virtually insoluble in water so that the reaction appears to go to completion. How many milliliters of 3.550 M Hl(aq) must be added to a solution containing 0.400 mol of Pb(NO3)2 (aq) to completely precipitate the lead? Select one: O a. 4.44'10-3 O b. 225 O c. 22.5 O d. 0.113 O e. 0.225 Which combination will produce a precipitate? Select one: O a. AgNO3 Ca(C2H302)2 (aq) 3 (aq) and O b. NaC2H3O2 (aq) and HCI (aq) C. NaOH and HCl (aq) (aq) d. KOH (aq) and Mg(NO3)2 (aq) O e. NaOH (aq) and HCl (aq) In which structure(s) below does not the oxygen have a formal charge of +1? HO: H-C=-H H3C_0CHE H-0-H 1 H CH3 IV Select one: O a. I and III O b. I only O c. I and IV O d. I, III, and IV O e. Il only The molecular geometry of the PF4 ion is Select one: a. None of these correct O b. trigonal bipyramidal O c. trigonal pyramidal O d. tetrahedral O e. octahedral A compound has a molecular formula of C12H2406. What is this compound's empirical formula? Select one: O a. C6H12O3 O b. CHO O c. C4H8O2 O d. C2H40 O e. C12H2406 What is the wavelength of electromagnetic radiation which has a frequency of 4.464 x 1014 s -1? Select one: a. 1.489 x 10-6 m O b. 6.716 x 10-7 nm O c. 1.338 x 1023 m O d. 671.6 nm O e. 7.472 x 10-15 nm The central atom in does not violate the octet rule. Select one: a. XeF4 O b. KrF2 O c. IC14- O d. SF4 O e. CF4 What is the wavelength of electromagnetic radiation which has a frequency of 4.464 x 1014 s -1? Select one: a. 1.489 x 10-6 m O b. 6.716 x 10-7 nm O c. 1.338 x 1023 m O d. 671.6 nm O e. 7.472 x 10-15 nm The central atom in does not violate the octet rule. Select one: O a. XeF4 O b. KrF2 O c. IC14- O d. SF4 O e. CF4 electrons appear in the following half-reaction when it is balanced. S406 2- 2- -> 25203 Select one: O a. 1 O b. 6 O c. 4 c4 d. 2 O e. 3 Based on the Aufbau principle and other applicable guiding principles, what ground state electronic configuration would one reasonably expect to find for technetium (Z = 43)? Select one: O a. [Kr] 4d7 O b. [Kr] 552 505 O c. [Kr] 452 405 O d. [Kr] 452 305 O e. [Kr] 552 405 Which of the following bonds exhibits the lowest ionic bond character? Select one: O a. H-F O b. H-Br O c. H-1 O d. H-CI O e. H-H Write the correct Lewis dot structure for CN. Which statement correctly describes the structure? Select one: O a. The structure contains 1 single bond and 6 lone pairs. O b b. The structure contains 1 double bond and 5 lone pairs. O c. The structure contains 1 double bond and 4 lone pairs. d. The structure contains 1 triple bond and 2 lone pairs. e. The structure contains 1 single bond and 7 lone pairs