Calculate the enthalpy and entropy of saturated isobutane vapor at 360 K from the following information: 1. Table 6.1 gives compressibility-factor data (values of Z)
Calculate the enthalpy and entropy of saturated isobutane vapor at 360 K from the following information:
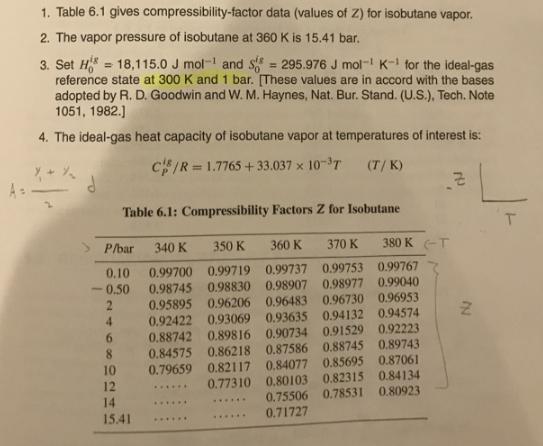
1. Table 6.1 gives compressibility-factor data (values of Z) for isobutane vapor. 2. The vapor pressure of isobutane at 360 K is 15.41 bar. 3. Set H = 18,115.0 J mol- and S reference state at 300 K and 1 bar. [These values are in accord with the bases adopted by R. D. Goodwin and W. M. Haynes, Nat. Bur. Stand. (U.S.), Tech. Note 1051, 1982.) 295.976 J mol- K- for the ideal-gas %3D 4. The ideal-gas heat capacity of isobutane vapor at temperatures of interest is: cIR = 1.7765 + 33.037 x 10-T (T/ K) Table 6.1: Compressibility Factors Z for Isobutane P/bar 340 K 350 K 360 K 370 K 380 K -T 0.10 0.99700 0.99719 0.99737 0.99753 0.99767 0.98745 0.98830 0.98907 0.98977 0.99040 0.95895 0.96206 0.96483 0.96730 0.96953 0.92422 0.93069 0.93635 0.94132 0.94574 0.88742 0.89816 0.90734 0.91529 0.92223 0.84575 0.86218 0.87586 0.88745 0.89743 0.79659 0.82117 0,84077 0.85695 0.87061 0.77310 0.80103 0.82315 0.84134 0.50 4. 8. 10 12 14 15.41 ...... 0.75506 0.78531 0.80923 ...... ...... 0.71727 ...... ......
Step by Step Solution
3.42 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
The sol...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


