Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the hardness of water in units of mg/L of CaCO3 (see equation 15-7) if your titration at pH = 10 resulted in a

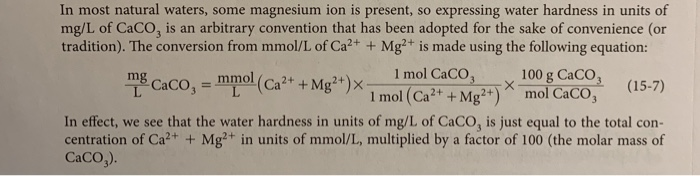
Calculate the hardness of water in units of mg/L of CaCO3 (see equation 15-7) if your titration at pH = 10 resulted in a concentration of 15 mmol/L. Round your answer to the nearest whole number and enter only the numerical answer into the box. In most natural waters, some magnesium ion is present, so expressing water hardness in units of mg/L of CaCO, is an arbitrary convention that has been adopted for the sake of convenience (or tradition). The conversion from mmol/L of Ca2+ + Mg2+ is made using the following equation: mg Caco, = mmol (Ca2+ + Mg2+)x- 1 mol CaCO3 1 mol (Ca2+ + Mg2+) 100 g CaCO3 mol CaCO3 (15-7) L In effect, we see that the water hardness in units of mg/L of CaCO, is just equal to the total con- centration of Ca2+ + Mg2+ in units of mmol/L, multiplied by a factor of 100 (the molar mass of CaCO3).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the hardness of water in units of mgL of CaCO3 we need to u...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
663de448b3b61_961288.pdf
180 KBs PDF File
663de448b3b61_961288.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


