Question
For this experiment, use the data shown below. Concentration of HCI (M) Initial Burette Reading Final Burette Reading Mass calorimeter cup (g) Mass calorimeter and
For this experiment, use the data shown below.
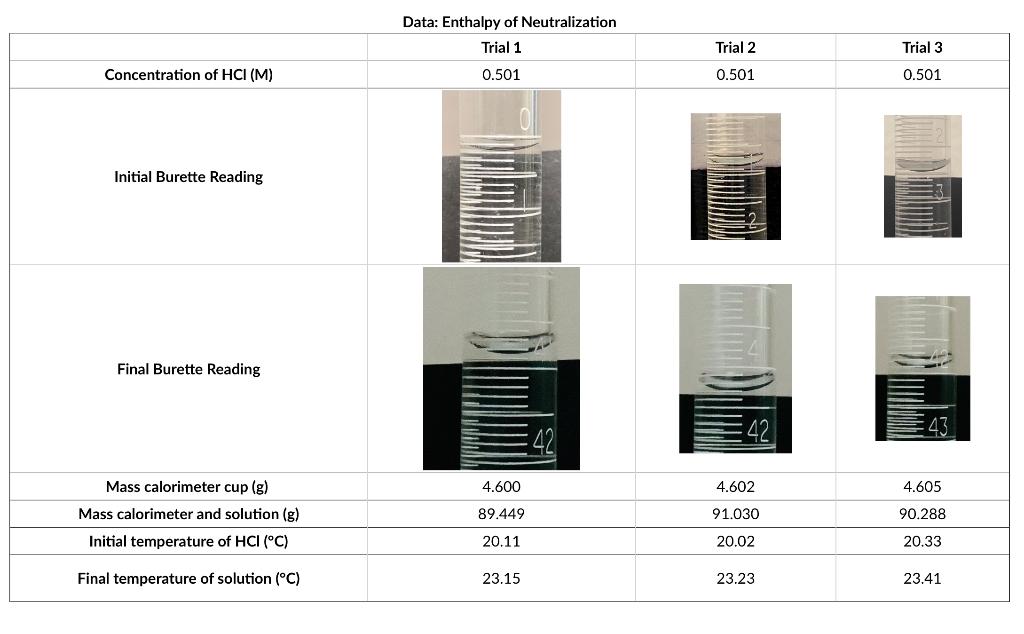



Concentration of HCI (M) Initial Burette Reading Final Burette Reading Mass calorimeter cup (g) Mass calorimeter and solution (g) Initial temperature of HCI (C) Final temperature of solution (C) Data: Enthalpy of Neutralization Trial 1 0.501 4.600 89.449 20.11 23.15 Trial 2 0.501 42 4.602 91.030 20.02 23.23 Trial 3 0.501 43 4.605 90.288 20.33 23.41
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Initial volume mL Final volume mL Mass of calorimeter cup g Mass of calorime...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of heat transfer
Authors: Frank Kreith, Raj M. Manglik, Mark S. Bohn
7th Edition
495667706, 978-0495667704
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



