Question
calculate the heat required to convert 15g of ice at -15 degrees Celsius into liquid water at 20 degrees Celsius. [10 pts] Calculate the
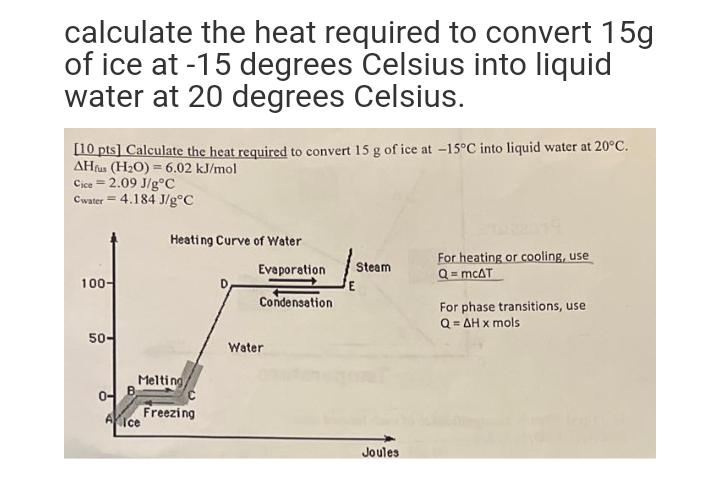
calculate the heat required to convert 15g of ice at -15 degrees Celsius into liquid water at 20 degrees Celsius. [10 pts] Calculate the heat required to convert 15 g of ice at -15C into liquid water at 20C. AHfus (HO) = 6.02 kJ/mol Cice=2.09 J/gC Cwater = 4.184 J/gC 100- 50- 0- Heating Curve of Water Melting Aice Freezing Evaporation Condensation Water E Steam Joules For heating or cooling, use Q = mcAT For phase transitions, use Q=AH x mols
Step by Step Solution
3.36 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



