Question
Calculate the molality of each of the following aqueous solutions. Part 1 of 2 2.51 M NaCl (density of solution = 1.08 Part 2
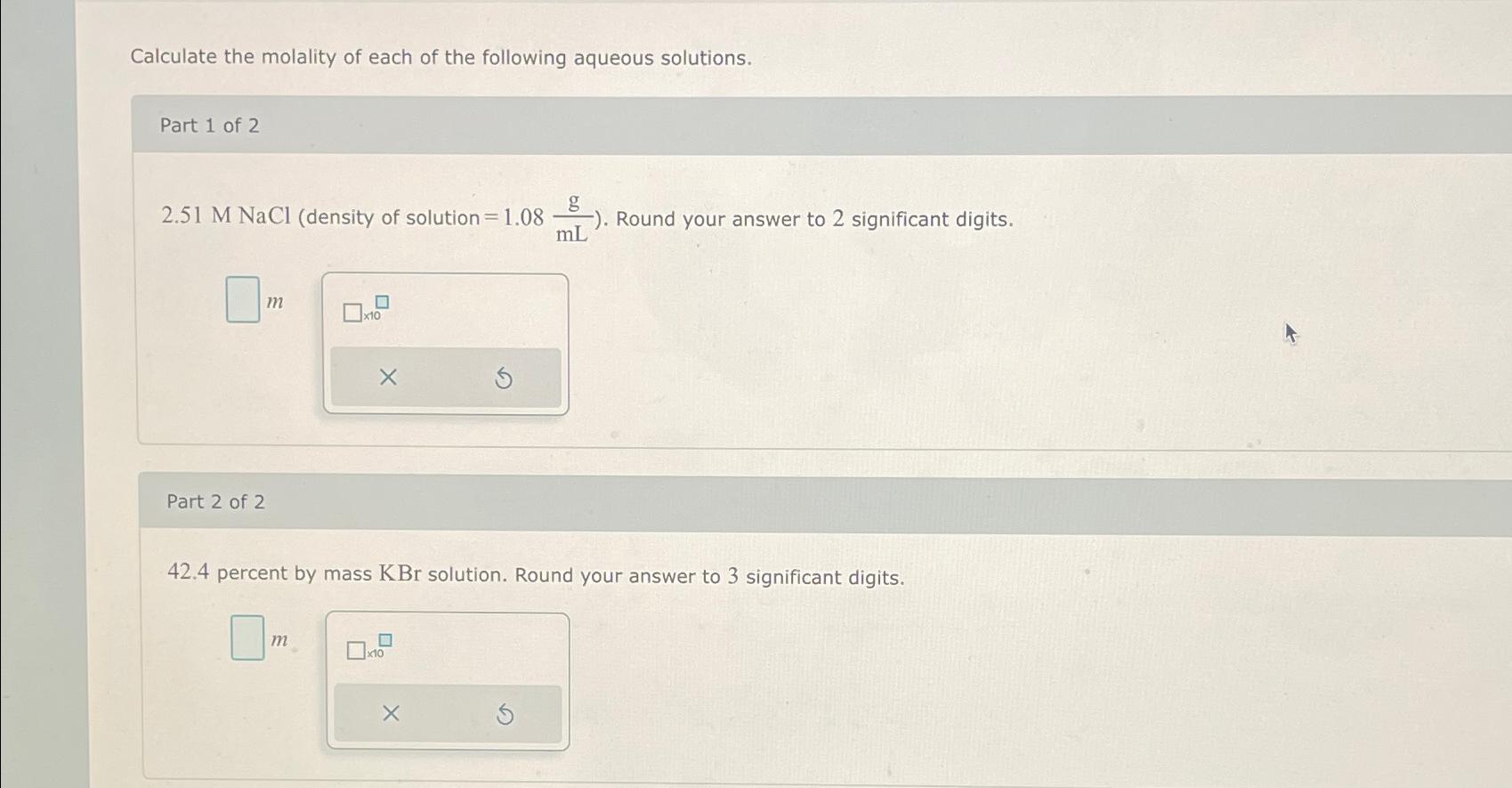
Calculate the molality of each of the following aqueous solutions. Part 1 of 2 2.51 M NaCl (density of solution = 1.08 Part 2 of 2 m 0 m x10 42.4 percent by mass KBr solution. Round your answer to 3 significant digits. x10 -). Round your answer to 2 significant digits. mL X
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Part 1 of 2 To calculate the molality of a 251 M NaCl aqueous solution with a density of 108 gmL we ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus And Its Applications
Authors: Larry Goldstein, David Lay, David Schneider, Nakhle Asmar
14th Edition
0134437772, 9780134437774
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



