Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the molar enthalpy change of methyl alcohol in the temperature range of 298.15 -473K at a pressure of 0.1013 MPa. The boiling point
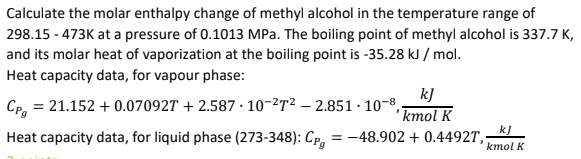
Calculate the molar enthalpy change of methyl alcohol in the temperature range of 298.15 -473K at a pressure of 0.1013 MPa. The boiling point of methyl alcohol is 337.7 K, and its molar heat of vaporization at the boiling point is -35.28 kJ/mol. Heat capacity data, for vapour phase: =21.152+0.07092T+2.587-10-272-2.851 10-8,- k] 'kmol K Heat capacity data, for liquid phase (273-348): Cpg = -48.902 + 0.4492T,; kJ kmol K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


