Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the moles of NaOHdelivered by using the volume of NaOH and themolarity of NaOH. (The volume needs to be in liters and theconcentration of
- Calculate the moles of NaOHdelivered by using the volume of NaOH and themolarity of NaOH. (The volume needs to be in liters and theconcentration of the NaOH is 1 M.)
- The moles of acetic acid insample is equal to the moles of NaOH deliveredbecause the acid has one H+ and the base has oneOH-. So it is a 1 to 1 ratio.
- The mass of acetic acid insample can be found by converting moles of aceticacid (HC2H3O2) to grams of aceticacid. (You may need to go back and review how to do molecalculations from unit 3.)
- The calculated molarity of acetic acid in thesampled vinegar is found using the molarity equation:moles of solute divided by liters of total solution.
- The calculated percent mass of acetic acid insampled vinegar is found by taking the mass of aceticacid in the sample divided by the mass of the vinegar andmultiplying by 100.
- Once all three trials are done, you can calculatethe average percent mass of acetic acid in sampledvinegar.
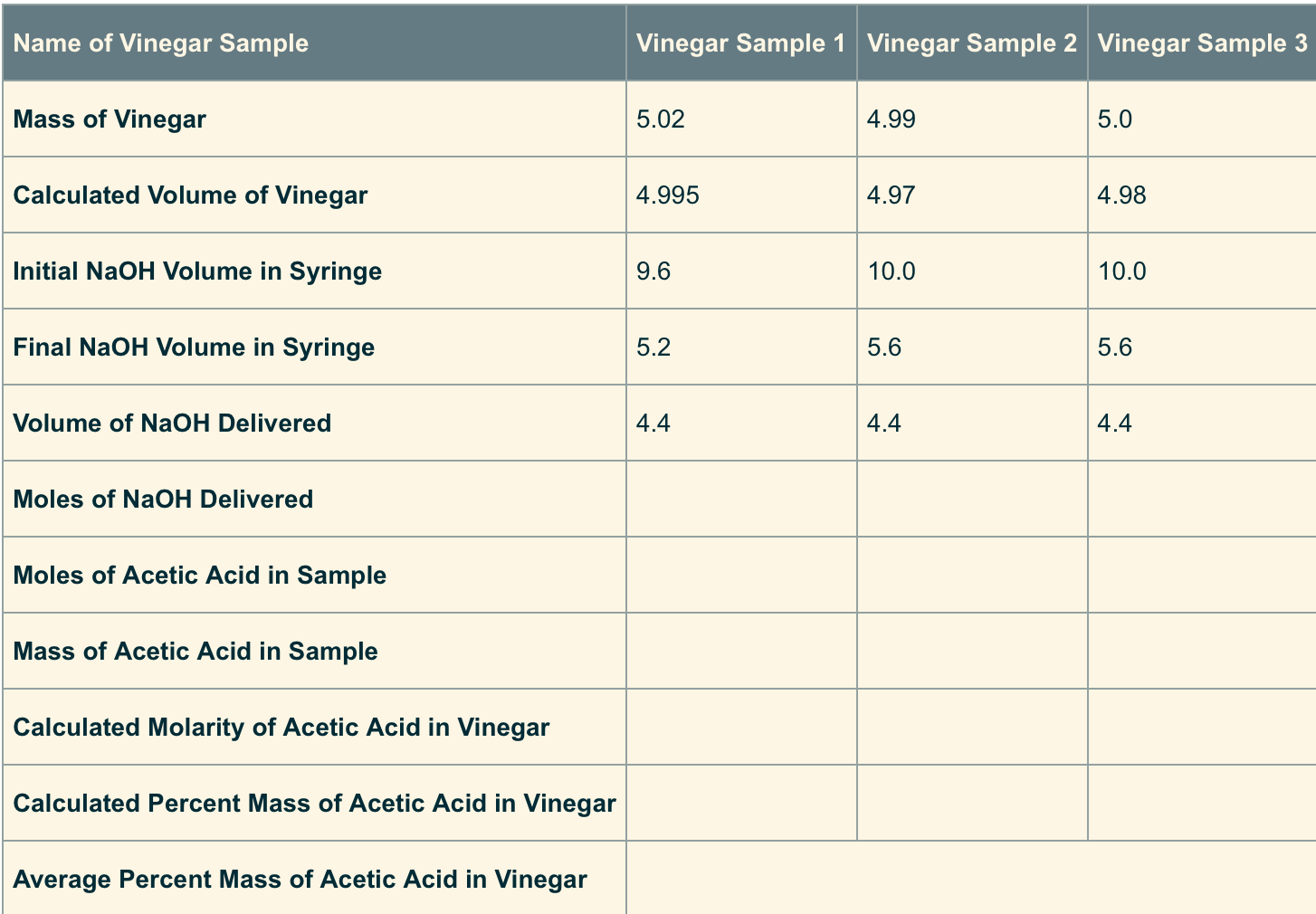
Name of Vinegar Sample Mass of Vinegar Calculated Volume of Vinegar Initial NaOH Volume in Syringe Final NaOH Volume in Syringe Volume of NaOH Delivered Moles of NaOH Delivered Moles of Acetic Acid in Sample Mass of Acetic Acid in Sample Calculated Molarity of Acetic Acid in Vinegar Calculated Percent Mass of Acetic Acid in Vinegar Average Percent Mass of Acetic Acid in Vinegar Vinegar Sample 1 Vinegar Sample 2 Vinegar Sample 3 5.02 4.995 9.6 5.2 4.4 4.99 4.97 10.0 5.6 4.4 5.0 4.98 10.0 5.6 4.4
Step by Step Solution
★★★★★
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


