Question
Calculate the percent purity of a sample of impure oxalic acid, HC2O4 2H2O if 0.4006 g of this material requires a titration with 28.62
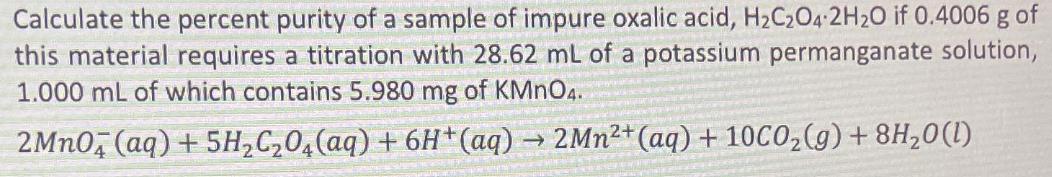
Calculate the percent purity of a sample of impure oxalic acid, HC2O4 2H2O if 0.4006 g of this material requires a titration with 28.62 mL of a potassium permanganate solution, 1.000 mL of which contains 5.980 mg of KMnO4. 2MnO4 (aq) + 5HCO4 (aq) + 6H+ (aq) 2Mn+ (aq) + 10C0(g) + 8H0 (1)
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the percent purity of the impure oxalic acid sample HCO2HO well first need to use the titration information provided to determine the amo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry And Chemical Reactivity
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
10th Edition
0357001176, 978-0357001172
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



