Question
Calculate the pH of a solution in which [H3O'] = 6.85x103 M. Give your answer to two places after the decimal. Part 2 (1
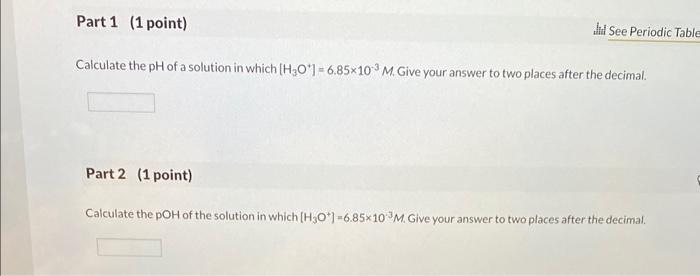

Calculate the pH of a solution in which [H3O'] = 6.85x103 M. Give your answer to two places after the decimal. Part 2 (1 point) Jd See Periodic Table Calculate the pOH of the solution in which [H3O*] -6.85x10M. Give your answer to two places after the decimal. Part 3 (1 point) If 100. mL of the solution in part 1 is concentrated by evaporation to give a final volume of 1.00 mL, what is the change in pl Choose one: pH decreases by 2. pH increases by 2. pH decreases by 10. pH increases by 10.
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Analytical Chemistry
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
9th edition
495558281, 978-0495558286
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



