Question
Calculate the pressure in kPa of 0.0773 mol of He at 393 K in a 10.59 L vessel. When writing out your answer, please
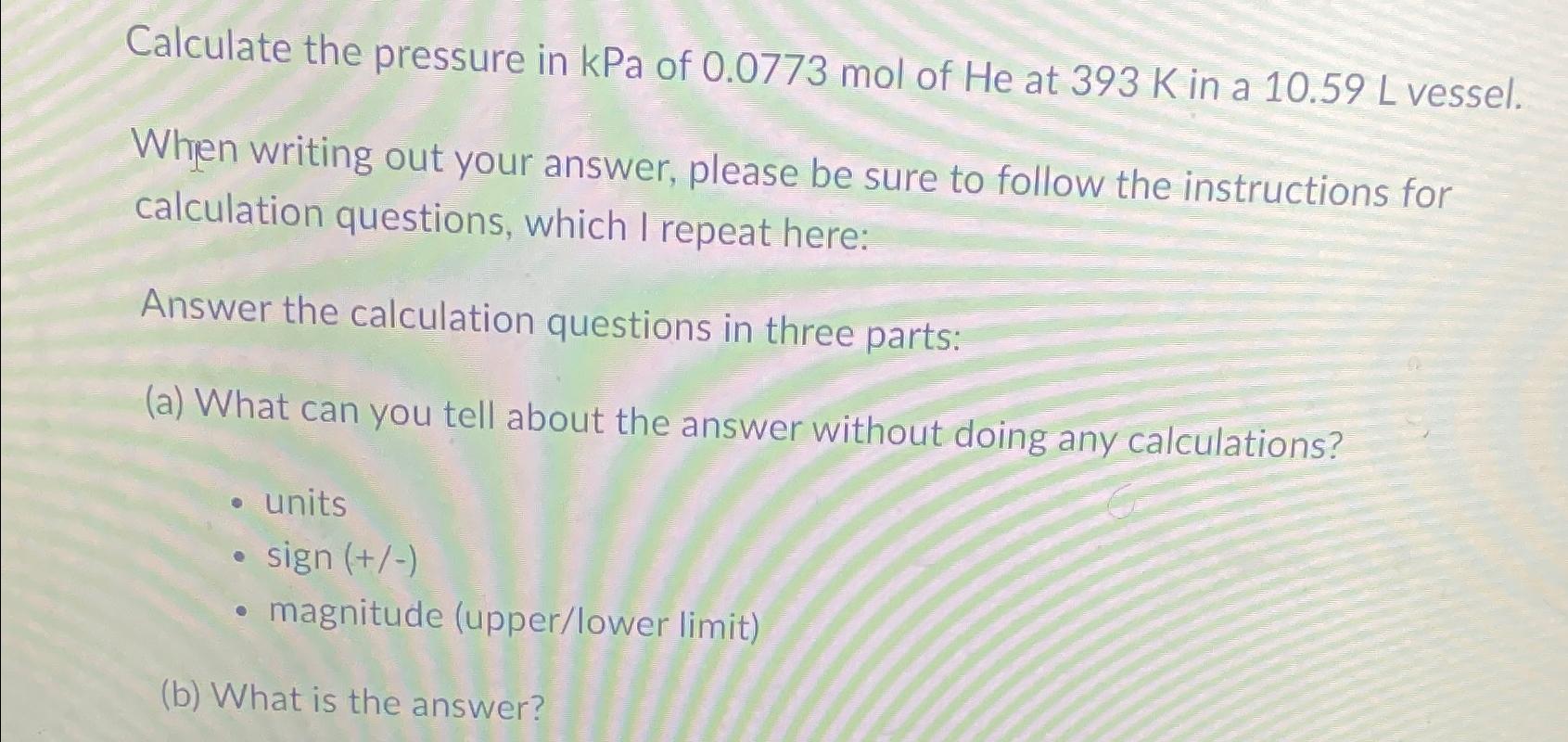
Calculate the pressure in kPa of 0.0773 mol of He at 393 K in a 10.59 L vessel. When writing out your answer, please be sure to follow the instructions for calculation questions, which I repeat here: Answer the calculation questions in three parts: (a) What can you tell about the answer without doing any calculations? units sign (+/-) magnitude (upper/lower limit) (b) What is the answer?
Step by Step Solution
3.42 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
The image you provided displays a question that asks for the calculation of pressure in kilopascals ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial accounting
Authors: ramji balakrishnan, k. s i varamakrishnan, Geoffrey b. sprin
1st edition
471467855, 978-0471467854
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



