Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the stopping power of a proton with an energy of 5MeV in water, using hand calculations. Please show all work. a. For this question,


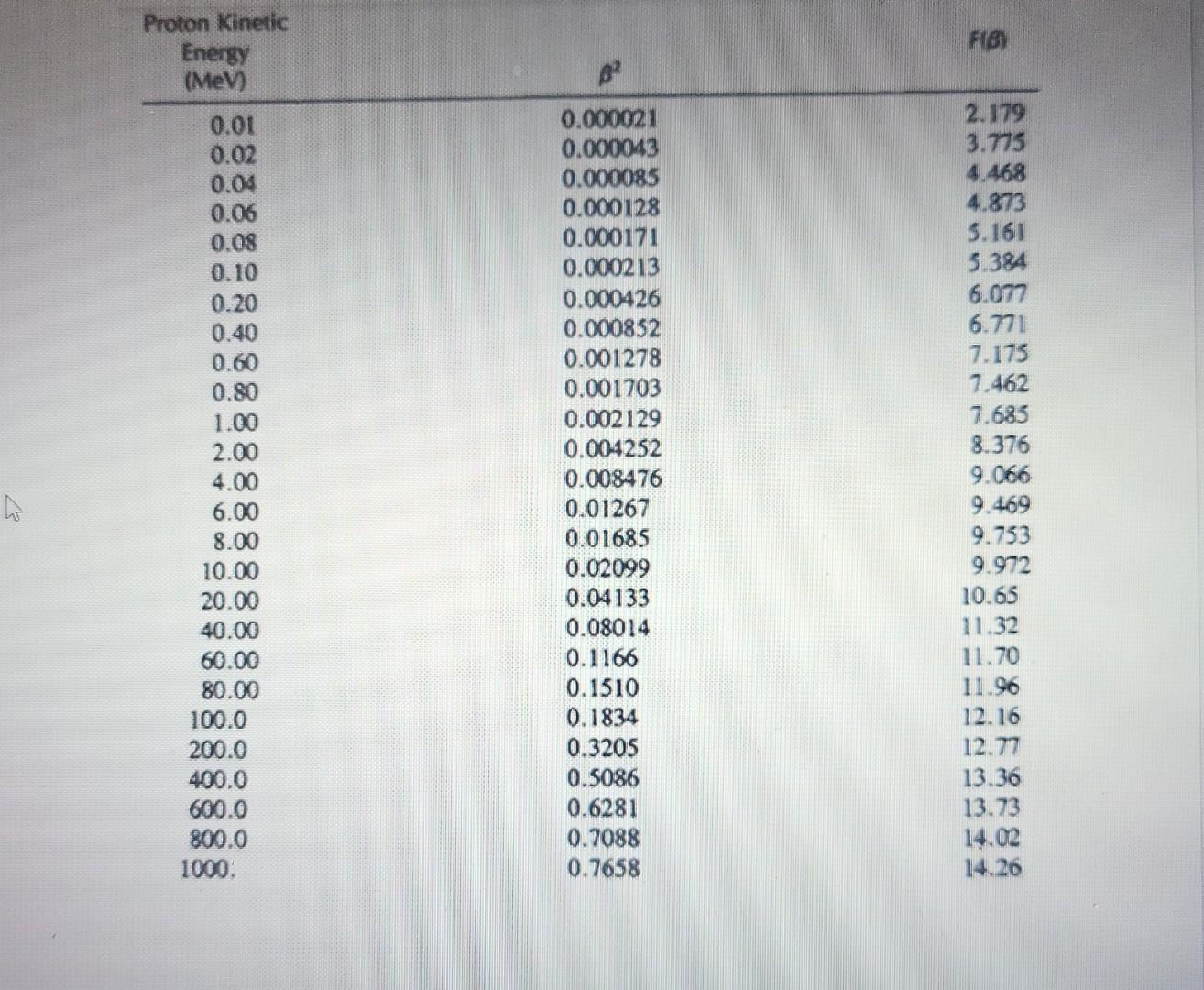
Calculate the stopping power of a proton with an energy of 5MeV in water, using hand calculations. Please show all work. a. For this question, use the following values for proton constants, which should be accessible, or given in the lecture content. Additional values can be found in Table 1 below. i. I=74.6eV ii. N=3.381023e/cm3 Considering the considerable difference in mass between an alpha particle and proton, calculate the stopping power of a 5MeV alpha using Table 1 and hand calculations Hint: consider the relationship between the particles for certain values. Proton Kinetic \begin{tabular}{rcc} Energy(MeV) & 2 & F() \\ \hline 0.01 & 0.000021 & 2.179 \\ 0.02 & 0.000043 & 3.775 \\ 0.04 & 0.000085 & 4.468 \\ 0.06 & 0.000128 & 4.873 \\ 0.08 & 0.000171 & 5.161 \\ 0.10 & 0.000213 & 5.384 \\ 0.20 & 0.000426 & 6.077 \\ 0.40 & 0.000852 & 6.771 \\ 0.60 & 0.001278 & 7.173 \\ 0.80 & 0.001703 & 7.462 \\ 1.00 & 0.002129 & 7.685 \\ 2.00 & 0.004252 & 8.376 \\ 4.00 & 0.008476 & 9.066 \\ 6.00 & 0.01267 & 9.469 \\ 8.00 & 0.01685 & 9.753 \\ 10.00 & 0.02099 & 9.972 \\ 20.00 & 0.04133 & 10.65 \\ 40.00 & 0.08014 & 11.32 \\ 60.00 & 0.1166 & 11.70 \\ 80.00 & 0.1510 & 11.96 \\ 100.0 & 0.1834 & 12.16 \\ 200.0 & 0.3205 & 12.77 \\ 400.0 & 0.5086 & 13.36 \\ 600.0 & 0.6281 & 13.73 \\ 800.0 & 0.7088 & 14.02 \\ 1000. & 0.7658 & 14.26 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


