Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the theoretical ratio between air and fuel during combustion of petrol (C7H13) in an engine (see picture of reaction below). Find: 1. Determine the
Calculate the theoretical ratio between air and fuel during combustion of petrol (C7H13) in an engine (see picture of reaction below).
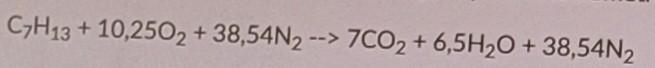
Find: 1. Determine the theoretical stoichiometric ratio (based on mass) between the air and the fuel.
2. How much CO2 ia released per kilometer (in grams CO2 per kilometer) when we assume that the car consumes 6.2 liters of petrol per 100 km (density of petrol= 0.75 kg/liter)?
3. In reality, the ratio (from 1) used is less then the calculated ratio, how does this affect the emissions from the combustion process?
C7H13+10,25O2+38,54N27CO2+6,5H2O+38,54N2Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


