Question
Calculate the water and salt flux of a cellulose acetate membrane used to desalinate a 5% NaCl solution at an operating pressure difference of
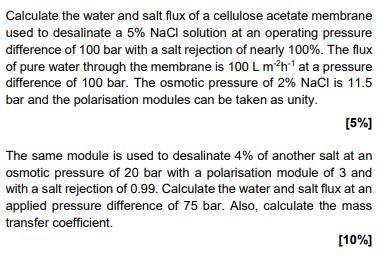
Calculate the water and salt flux of a cellulose acetate membrane used to desalinate a 5% NaCl solution at an operating pressure difference of 100 bar with a salt rejection of nearly 100%. The flux of pure water through the membrane is 100 L m2h1 at a pressure difference of 100 bar. The osmotic pressure of 2% NaCl is 11.5 bar and the polarisation modules can be taken as unity. [5%] The same module is used to desalinate 4% of another salt at an osmotic pressure of 20 bar with a polarisation module of 3 and with a salt rejection of 0.99. Calculate the water and salt flux at an applied pressure difference of 75 bar. Also, calculate the mass transfer coefficient. [10%]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Lets solve the provided membrane desalination problems step by step Part 1 Calculation of Water and Salt Flux for a 5 NaCl Solution Given Operating pr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Statistics And Probability For Engineers
Authors: Douglas C. Montgomery, George C. Runger
6th Edition
1118539710, 978-1118539712
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



