Question
Calculate the wavelength ???? and the frequency ???? of the photonsthat have an energy of ????photon=1.32?10?19 J. Use ????=3.00?108 m/s forthe speed of light in
Calculate the wavelength ???? and the frequency ???? of the photonsthat have an energy of ????photon=1.32?10?19 J. Use ????=3.00?108 m/s forthe speed of light in a vacuum.
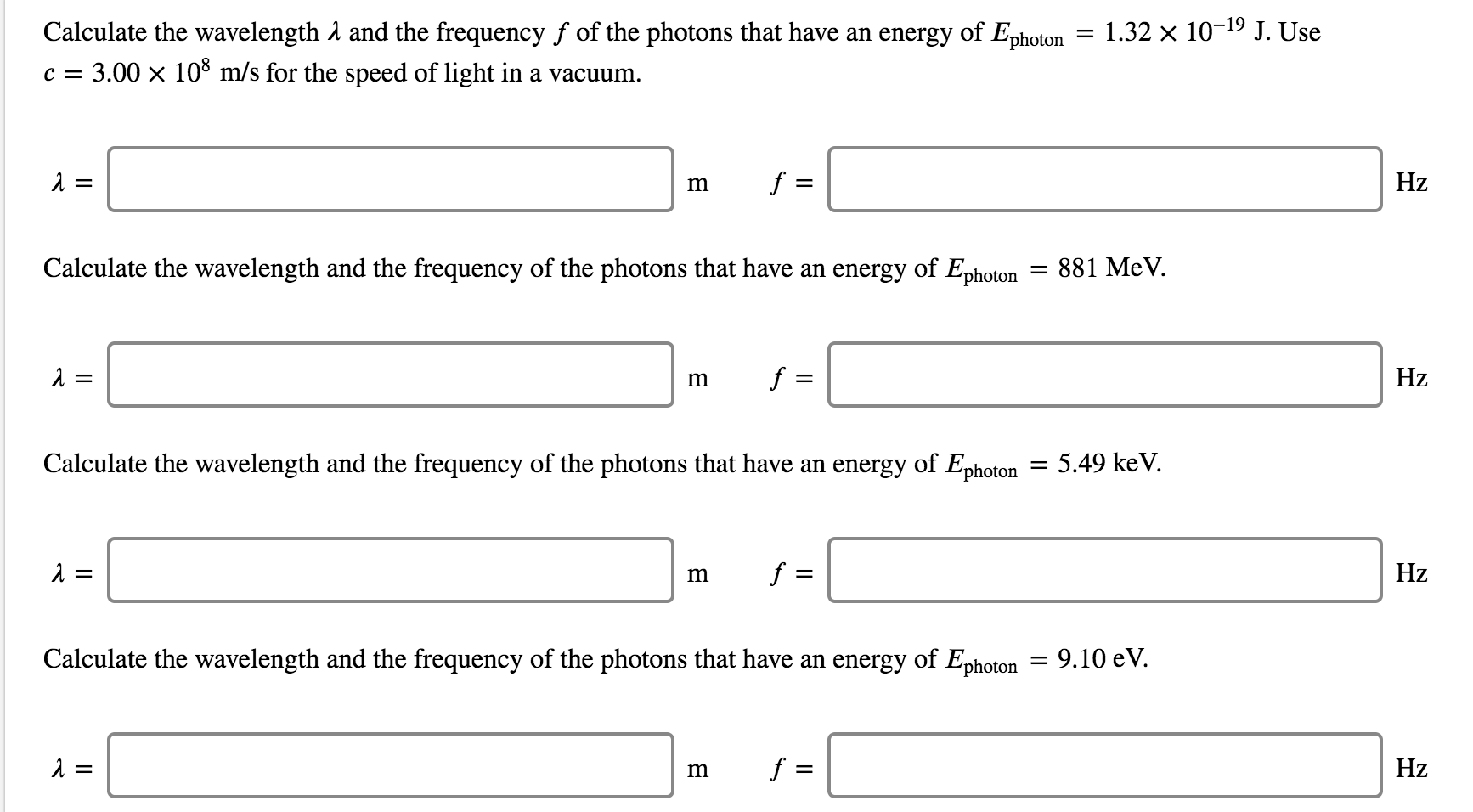
Calculate the wavelength and the frequency f of the photons that have an energy of Ephoton c = 3.00 108 m/s for the speed of light in a vacuum. = = Calculate the wavelength and the frequency of the photons that have an energy of Ephoton m = f = Calculate the wavelength and the frequency of the photons that have an energy of Ephoton = m f = m f = m f = - = 1.32 10-1 J. Use = 881 MeV. Calculate the wavelength and the frequency of the photons that have an energy of Ephoton = 9.10 eV. 5.49 keV. Hz Hz Hz Hz
Step by Step Solution
3.58 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Answer Phetku E he planpes enstand h 662610 122x10195 d 34 X 1 P x hf f f f frequency of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: James S. Walker
5th edition
978-0133498493, 9780321909107, 133498492, 0321909100, 978-0321976444
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



