Answered step by step
Verified Expert Solution
Question
1 Approved Answer
calculate z and molar volume for methane at the following conditions 2. Special problem using SOPE and NIST-Demo in class Use SOPE with the following
calculate z and molar volume for methane at the following conditions
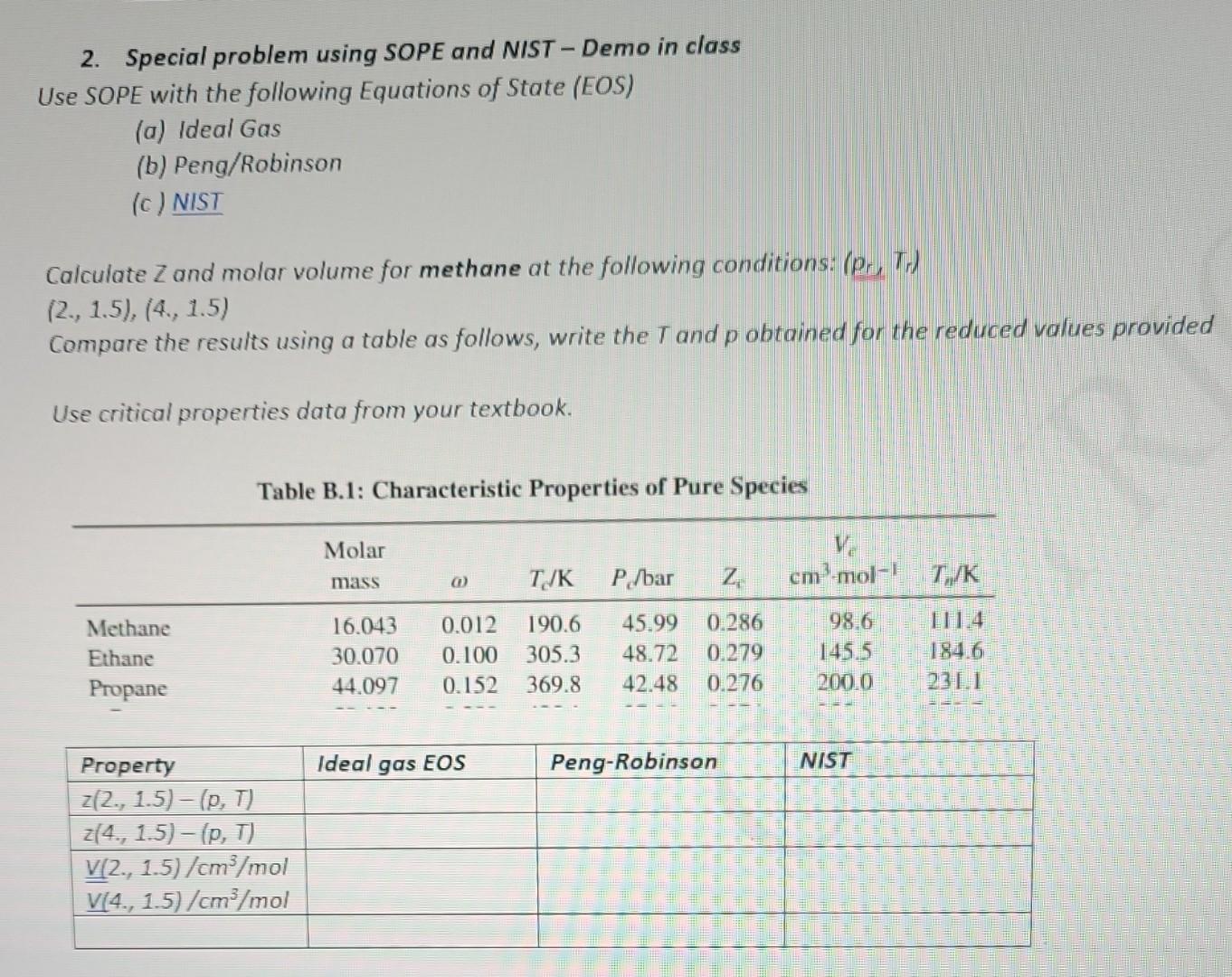
2. Special problem using SOPE and NIST-Demo in class Use SOPE with the following Equations of State (EOS) (a) Ideal Gas (b) Peng/Robinson (c) NIST Calculate Z and molar volume for methane at the following conditions: (pr,Tr) (2., 1.5), (4., 1.5) Compare the results using a table as follows, write the T and p obtained for the reduced values provided Use critical properties data from your textbook. Table B.1: Characteristic Properties of Pure Species
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


