Answered step by step
Verified Expert Solution
Question
1 Approved Answer
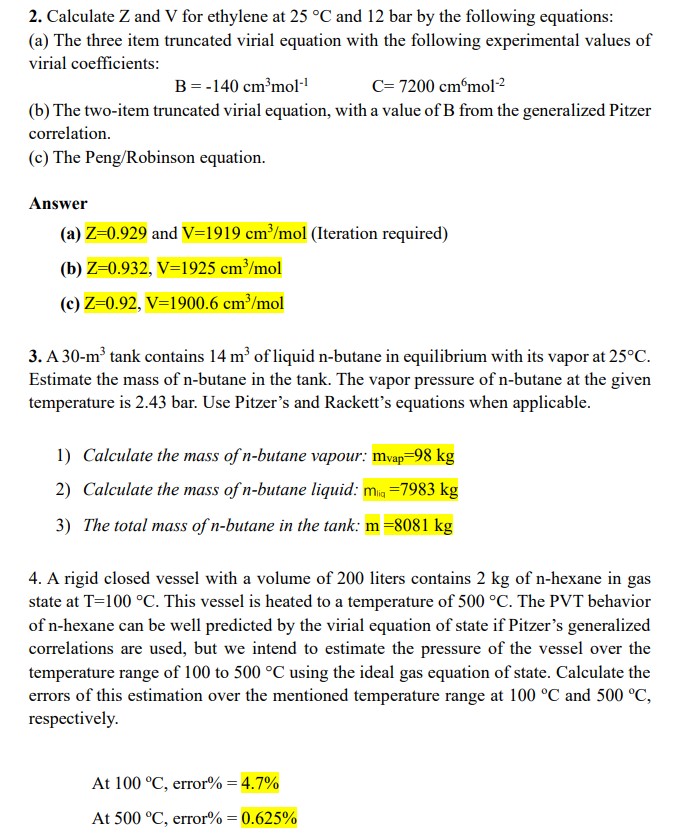
Calculate Z and V for ethylene at 2 5 C and 1 2 bar by the following equations: ( a ) The three item truncated
Calculate and for ethylene at and bar by the following equations:
a The three item truncated virial equation with the following experimental values of
virial coefficients:
b The twoitem truncated virial equation, with a value of from the generalized Pitzer
correlation.
c The PengRobinson equation.
Answer
a and Iteration required
b
c
A tank contains of liquid butane in equilibrium with its vapor at
Estimate the mass of nbutane in the tank. The vapor pressure of butane at the given
temperature is bar. Use Pitzer's and Rackett's equations when applicable.
Calculate the mass of nbutane vapour:
Calculate the mass of nbutane liquid:
The total mass of nbutane in the tank:
A rigid closed vessel with a volume of liters contains of hexane in gas
state at This vessel is heated to a temperature of The PVT behavior
of nhexane can be well predicted by the virial equation of state if Pitzer's generalized
correlations are used, but we intend to estimate the pressure of the vessel over the
temperature range of to using the ideal gas equation of state. Calculate the
errors of this estimation over the mentioned temperature range at and
respectively.
At error
At error
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


