Answered step by step
Verified Expert Solution
Question
1 Approved Answer
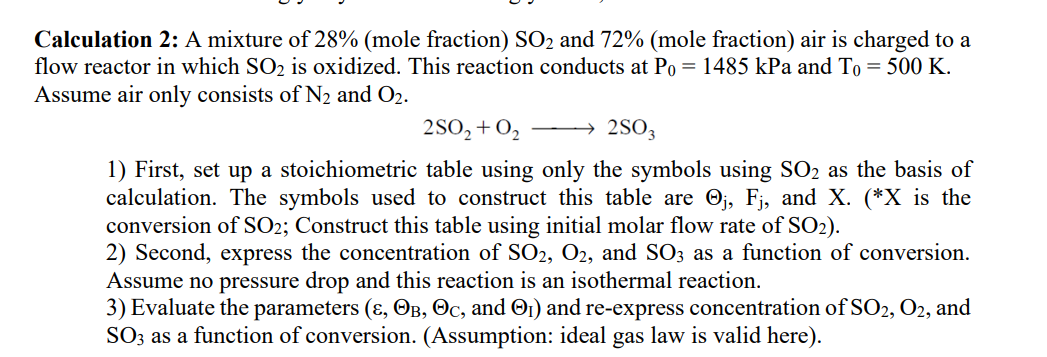
Calculation 2 : A mixture of 2 8 % ( mole fraction ) S O 2 and 7 2 % ( mole fraction ) air
Calculation : A mixture of mole fraction and mole fraction air is charged to a
flow reactor in which is oxidized. This reaction conducts at kPa and
Assume air only consists of and
longrightarrow
First, set up a stoichiometric table using only the symbols using as the basis of
calculation. The symbols used to construct this table are and is the
conversion of ; Construct this table using initial molar flow rate of
Second, express the concentration of and as a function of conversion.
Assume no pressure drop and this reaction is an isothermal reaction.
Evaluate the parameters and and reexpress concentration of and
as a function of conversion. Assumption: ideal gas law is valid here
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


