
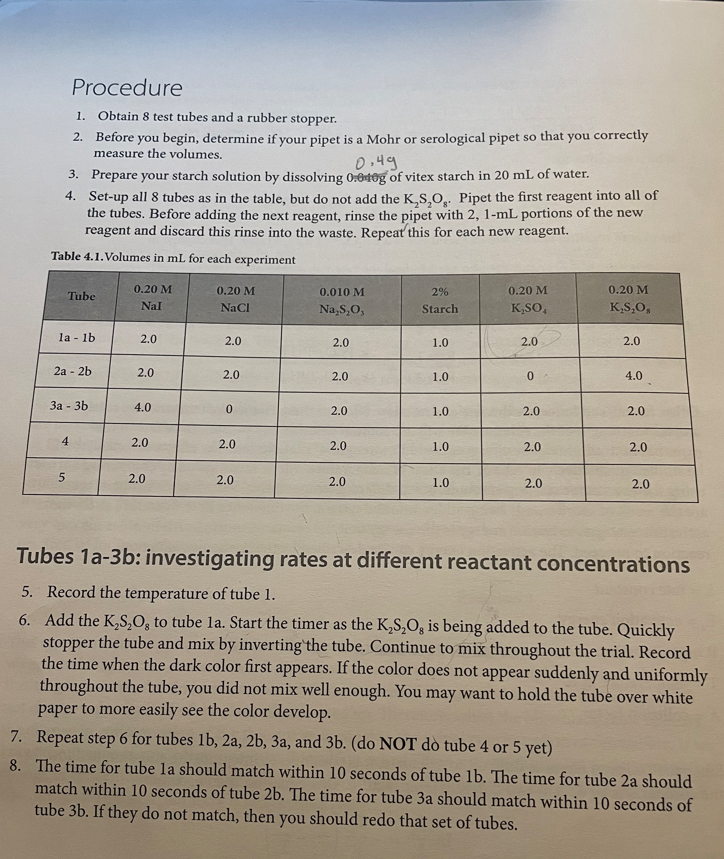
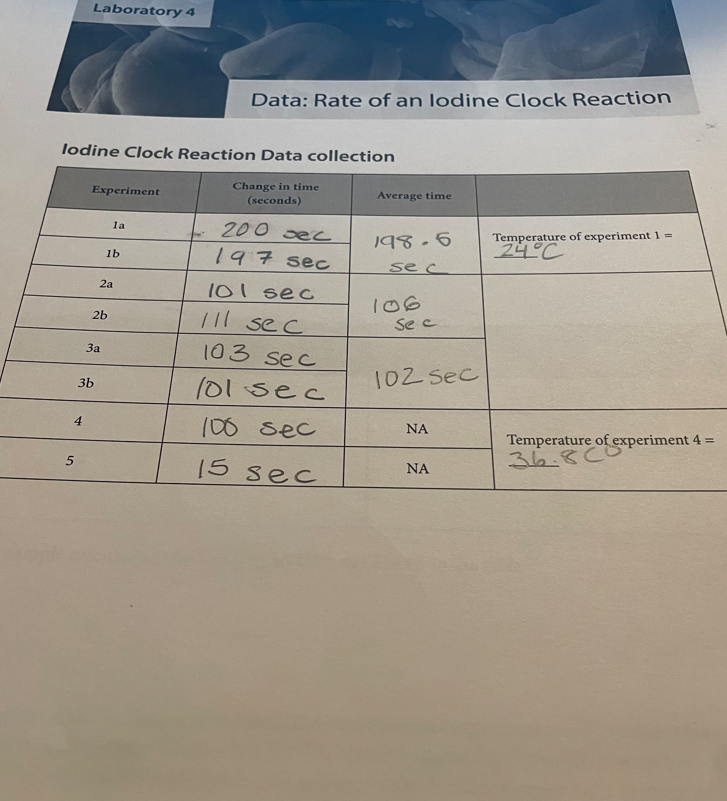
Calculation and Questions 1. Calculate the initial rate; [S2O82]/t, for experiments 1,2,3 and 4. Remember that the rate with respect to S2O822 can't be calculated directly from the initial concentration of S2O82. It was only measurable because the iodine reacted away the S2O32 and began to accumulate. 2. Fill in the table below. Show sample calculations for [S2O82]0 and [I]0 used to fill in the table. 3. Using the data for the room temperature experiments (experiments 1-3) in the table in question 2, determine the rate law for this reaction including the rate constant with appropriate units. The rate constant should be the average of the 3 room temperature experiments. Laboratory 4 | The Rate of an lodine Clock Reaction 4 4. Use the rate law to quantitatively explain what would happen in the following situations: a. What would happen to the rate of the reaction if the Iodide concentration was doubled? b. What would happen to the rate of the reaction if the persulfate concentration was halved? c. What would happen to the rate of the reaction if the thiosulfate concentration was doubled? d. How would increasing the total volume of the solution affect the actual rate of the iodine clock reaction? 5. Calculate the rate constant at the higher temperature (tube 4). 6. Calculate the activation energy for the reaction. 7. Describe the effect that the catalyst had on the rate of the reaction. Procedure 1. Obtain 8 test tubes and a rubber stopper. 2. Before you begin, determine if your pipet is a Mohr or serological pipet so that you correctly measure the volumes. 3. Prepare your starch solution by dissolving 0=0,0g of vitex starch in 20mL of water. 4. Set-up all 8 tubes as in the table, but do not add the K2S2O8. Pipet the first reagent into all of the tubes. Before adding the next reagent, rinse the pipet with 2, 1-mL portions of the new reagent and discard this rinse into the waste. Repeat this for each new reagent. Table 4.1. Volumes in mL for each experiment Tubes 1a-3b: investigating rates at different reactant concentrations 5. Record the temperature of tube 1 . 6. Add the K2S2O8 to tube 1a. Start the timer as the K2S2O8 is being added to the tube. Quickly stopper the tube and mix by inverting the tube. Continue to mix throughout the trial. Record the time when the dark color first appears. If the color does not appear suddenly and uniformly throughout the tube, you did not mix well enough. You may want to hold the tube over white paper to more easily see the color develop. 7. Repeat step 6 for tubes 1b,2a,2b,3a, and 3b. (do NOT do tube 4 or 5 yet) 8. The time for tube la should match within 10 seconds of tube 1b. The time for tube 2 a should match within 10 seconds of tube 2b. The time for tube 3 a should match within 10 seconds of tube 3b. If they do not match, then you should redo that set of tubes. lodine Clock Reaction Data collection Calculation and Questions 1. Calculate the initial rate; [S2O82]/t, for experiments 1,2,3 and 4. Remember that the rate with respect to S2O822 can't be calculated directly from the initial concentration of S2O82. It was only measurable because the iodine reacted away the S2O32 and began to accumulate. 2. Fill in the table below. Show sample calculations for [S2O82]0 and [I]0 used to fill in the table. 3. Using the data for the room temperature experiments (experiments 1-3) in the table in question 2, determine the rate law for this reaction including the rate constant with appropriate units. The rate constant should be the average of the 3 room temperature experiments. Laboratory 4 | The Rate of an lodine Clock Reaction 4 4. Use the rate law to quantitatively explain what would happen in the following situations: a. What would happen to the rate of the reaction if the Iodide concentration was doubled? b. What would happen to the rate of the reaction if the persulfate concentration was halved? c. What would happen to the rate of the reaction if the thiosulfate concentration was doubled? d. How would increasing the total volume of the solution affect the actual rate of the iodine clock reaction? 5. Calculate the rate constant at the higher temperature (tube 4). 6. Calculate the activation energy for the reaction. 7. Describe the effect that the catalyst had on the rate of the reaction. Procedure 1. Obtain 8 test tubes and a rubber stopper. 2. Before you begin, determine if your pipet is a Mohr or serological pipet so that you correctly measure the volumes. 3. Prepare your starch solution by dissolving 0=0,0g of vitex starch in 20mL of water. 4. Set-up all 8 tubes as in the table, but do not add the K2S2O8. Pipet the first reagent into all of the tubes. Before adding the next reagent, rinse the pipet with 2, 1-mL portions of the new reagent and discard this rinse into the waste. Repeat this for each new reagent. Table 4.1. Volumes in mL for each experiment Tubes 1a-3b: investigating rates at different reactant concentrations 5. Record the temperature of tube 1 . 6. Add the K2S2O8 to tube 1a. Start the timer as the K2S2O8 is being added to the tube. Quickly stopper the tube and mix by inverting the tube. Continue to mix throughout the trial. Record the time when the dark color first appears. If the color does not appear suddenly and uniformly throughout the tube, you did not mix well enough. You may want to hold the tube over white paper to more easily see the color develop. 7. Repeat step 6 for tubes 1b,2a,2b,3a, and 3b. (do NOT do tube 4 or 5 yet) 8. The time for tube la should match within 10 seconds of tube 1b. The time for tube 2 a should match within 10 seconds of tube 2b. The time for tube 3 a should match within 10 seconds of tube 3b. If they do not match, then you should redo that set of tubes. lodine Clock Reaction Data collection










