Question
The observed lines in the hydrogen spectrum all arise from jumps of electrons from one energy level to another. The wavelengths in nanometers corresponding
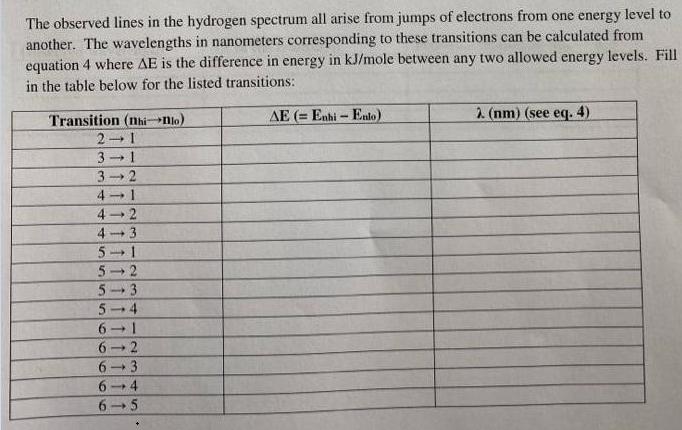
The observed lines in the hydrogen spectrum all arise from jumps of electrons from one energy level to another. The wavelengths in nanometers corresponding to these transitions can be calculated from equation 4 where AE is the difference in energy in kJ/mole between any two allowed energy levels. Fill in the table below for the listed transitions: AE (= Enhi - Ento) 2 (nm) (see eq. 4). Transition (nhiNlo) 2 1 3 1 3 2 41 4- 2 4- 3 5 1 5 2 5- 3 5 4 6 2 6. 3 6. 4 6.
Step by Step Solution
3.56 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics for Scientists and Engineers A Strategic Approach with Modern Physics
Authors: Randall D. Knight
4th edition
978-0134092508, 134092503, 133942651, 978-0133942651
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



