Answered step by step
Verified Expert Solution
Question
1 Approved Answer
CALCULATIONS Calculations for Reaction Order and Rate Constant Determination Calculating the reaction rate For each experiment, (show your work for experiment 2) 1. Calculate the

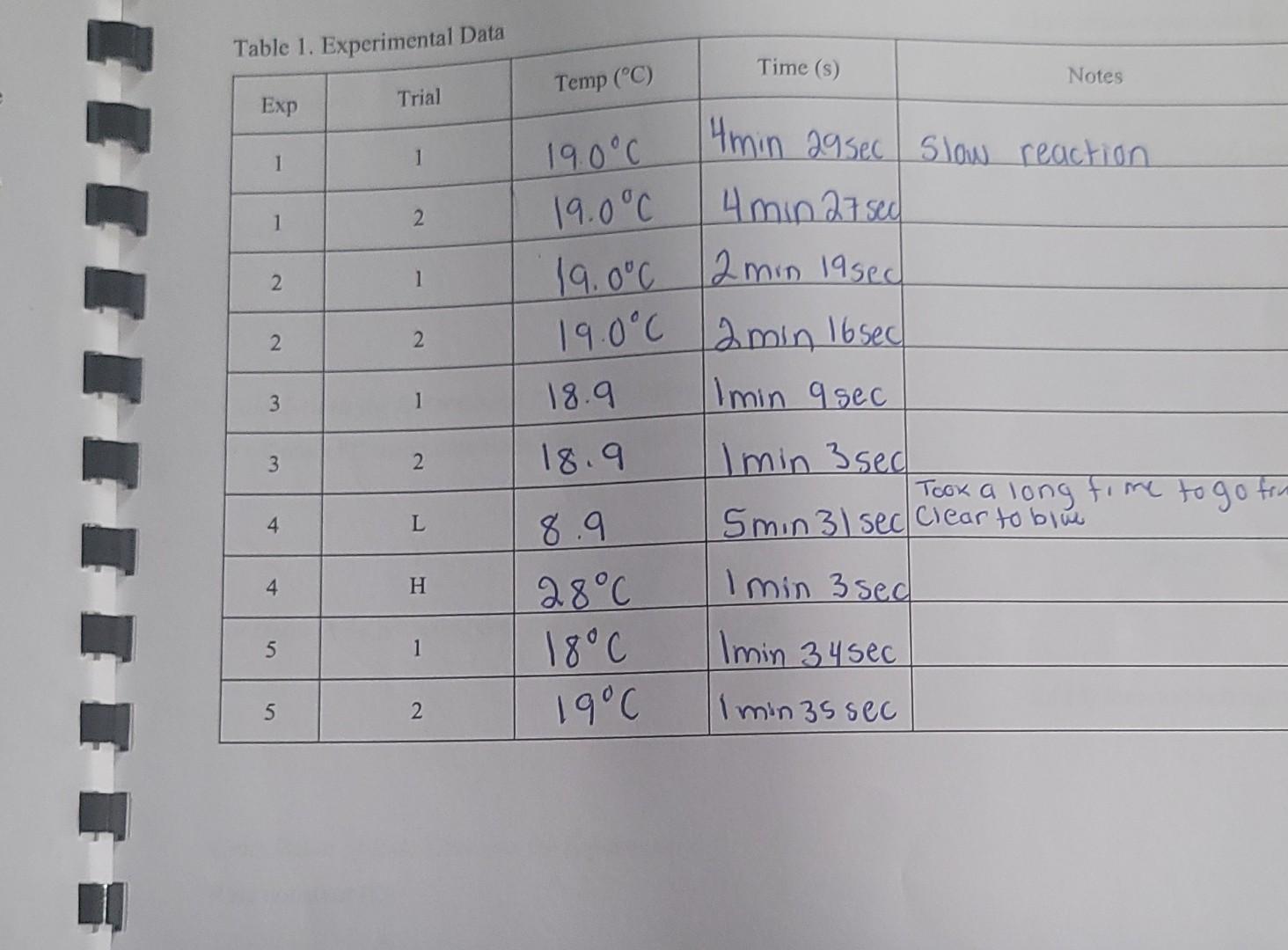

CALCULATIONS Calculations for Reaction Order and Rate Constant Determination Calculating the reaction rate For each experiment, (show your work for experiment 2) 1. Calculate the millimoles of thiosulfate added to the reaction and use the stoichiometry of reaction 2 to convert the value to millimoles of iodine. 2. Calculate the molarity change of iodine. 3. Calculate the reaction rate from the moles of iodine and the reaction time. 4. Calculate the average reaction rate for each experiment. Table 1. Experimental Data Table 1. Experimental Data \begin{tabular}{|c|c|c|c|c} \hline Exp & Trial & Temp (C) & Time (s) & \multicolumn{1}{|c|}{ Notes } \\ \hline 1 & 1 & 190C & 4min29sec & Slow reaction \\ \hline 1 & 2 & 19.0C & 4min27sec & \\ \hline 2 & 1 & 19.0C & 2min19sec \\ \hline 2 & 2 & 19.0C & 2min16sec & \\ \hline 3 & 1 & 18.9 & Imin9sec & \\ \hline 3 & 2 & 18.9 & 1min3sec & \\ \hline 4 & L & 8.9 & 5min31sec & Took a long ficar to blwe to go trin \\ \hline 4 & H & 28C & 1min3sec & \\ \hline 5 & 1 & 18C & Imin34sec & \\ \hline 5 & 2 & 19C & Imin35sec & \\ \hline \end{tabular} Calculations for Reaction Order and Rate Constant Determination Rate Calculations for Experiment 2 Total reaction volume (mL) : mmolS2O32 mmolI2 reacted: [I2] Average time (s): Average reaction rate (M/s) : CALCULATIONS Calculations for Reaction Order and Rate Constant Determination Calculating the reaction rate For each experiment, (show your work for experiment 2) 1. Calculate the millimoles of thiosulfate added to the reaction and use the stoichiometry of reaction 2 to convert the value to millimoles of iodine. 2. Calculate the molarity change of iodine. 3. Calculate the reaction rate from the moles of iodine and the reaction time. 4. Calculate the average reaction rate for each experiment. Table 1. Experimental Data Table 1. Experimental Data \begin{tabular}{|c|c|c|c|c} \hline Exp & Trial & Temp (C) & Time (s) & \multicolumn{1}{|c|}{ Notes } \\ \hline 1 & 1 & 190C & 4min29sec & Slow reaction \\ \hline 1 & 2 & 19.0C & 4min27sec & \\ \hline 2 & 1 & 19.0C & 2min19sec \\ \hline 2 & 2 & 19.0C & 2min16sec & \\ \hline 3 & 1 & 18.9 & Imin9sec & \\ \hline 3 & 2 & 18.9 & 1min3sec & \\ \hline 4 & L & 8.9 & 5min31sec & Took a long ficar to blwe to go trin \\ \hline 4 & H & 28C & 1min3sec & \\ \hline 5 & 1 & 18C & Imin34sec & \\ \hline 5 & 2 & 19C & Imin35sec & \\ \hline \end{tabular} Calculations for Reaction Order and Rate Constant Determination Rate Calculations for Experiment 2 Total reaction volume (mL) : mmolS2O32 mmolI2 reacted: [I2] Average time (s): Average reaction rate (M/s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


