Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can Answer all the question? a. Consider the following chemical reaction between component A and B: A (9) + 2B (g) C(g) + 3D (1)
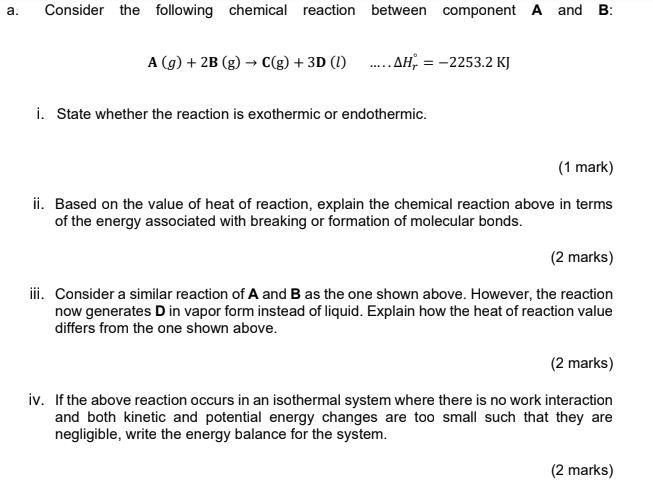
Can Answer all the question?
a. Consider the following chemical reaction between component A and B: A (9) + 2B (g) C(g) + 3D (1) ..... AH = -2253.2 KJ i. State whether the reaction is exothermic or endothermic. (1 mark) ii. Based on the value of heat of reaction, explain the chemical reaction above in terms of the energy associated with breaking or formation of molecular bonds. (2 marks) iii. Consider a similar reaction of A and B as the one shown above. However, the reaction now generates Din vapor form instead of liquid. Explain how the heat of reaction value differs from the one shown above. (2 marks) iv. If the above reaction occurs in an isothermal system where there is no work interaction and both kinetic and potential energy changes are too small such that they are negligible, write the energy balance for the system. (2 marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


