Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can i get the mass of NO in kg please Calculate the mass of NO produced. Hint Using multiple attempts will impact your score. 5%
can i get the mass of NO in kg please 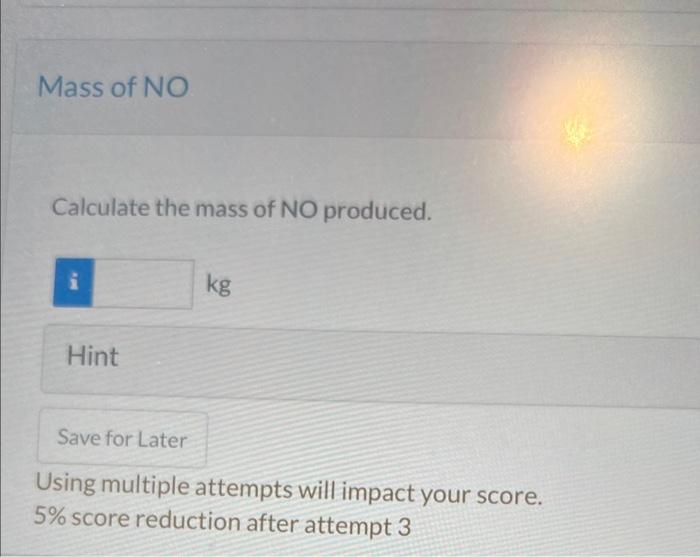
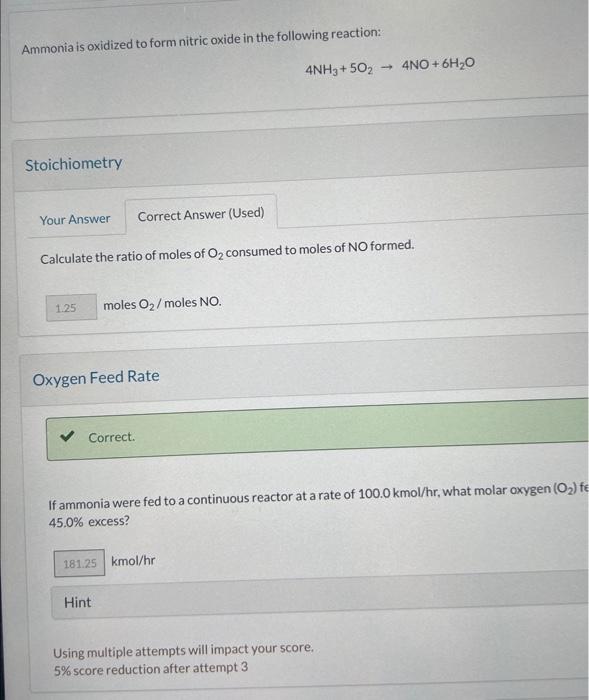
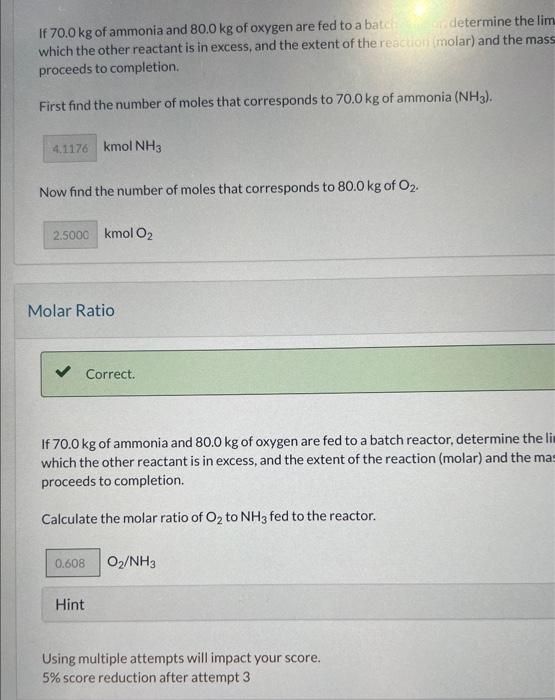
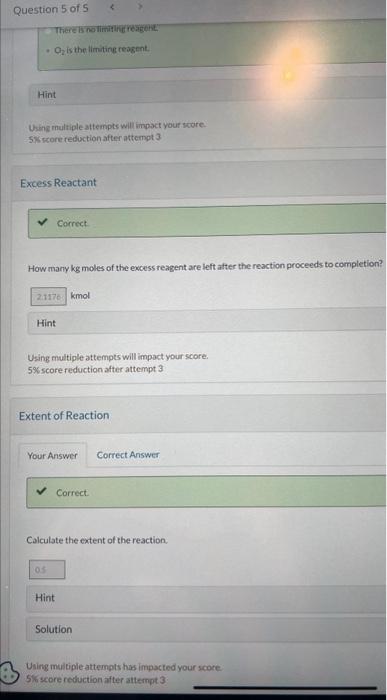 Calculate the mass of NO produced. Hint Using multiple attempts will impact your score. 5% score reduction after attempt 3 Ammonia is oxidized to form nitric oxide in the following reaction: 4NH3+5O24NO+6H2O Stoichiometry Calculate the ratio of moles of O2 consumed to moles of NO formed. moles O2/ moles NO Oxygen Feed Rate If ammonia were fed to a continuous reactor at a rate of 100.0kmol/hr, what molar oxygen (O2)f 45.0% excess? kmol/hr Hint Using multiple attempts will impact your score. 5% score reduction after attempt 3 If 70.0kg of ammonia and 80.0kg of oxygen are fed to a batcichine the lim which the other reactant is in excess, and the extent of the reaction (molar) and the mass proceeds to completion. First find the number of moles that corresponds to 70.0kg of ammonia (NH3). kmolNH3 Now find the number of moles that corresponds to 80.0kg of O2. kmolO2 Molar Ratio Correct. If 70.0kg of ammonia and 80.0kg of oxygen are fed to a batch reactor, determine the li which the other reactant is in excess, and the extent of the reaction (molar) and the ma: proceeds to completion. Calculate the molar ratio of O2 to NH3 fed to the reactor. O2/NH3 Using multiple attempts will impact your score. 5% score reduction after attempt 3 How many kg moles of the excess reagent are left atter the reaction proceeds to completion? kmol Hint Using multiple attempts will impact your score. 5% score reduction after attempt 3 Extent of Reaction Colculate the extent of the reaction. Hint Solution Uning multiple attempts has impacted your score. 5) score reduction after attempt 3
Calculate the mass of NO produced. Hint Using multiple attempts will impact your score. 5% score reduction after attempt 3 Ammonia is oxidized to form nitric oxide in the following reaction: 4NH3+5O24NO+6H2O Stoichiometry Calculate the ratio of moles of O2 consumed to moles of NO formed. moles O2/ moles NO Oxygen Feed Rate If ammonia were fed to a continuous reactor at a rate of 100.0kmol/hr, what molar oxygen (O2)f 45.0% excess? kmol/hr Hint Using multiple attempts will impact your score. 5% score reduction after attempt 3 If 70.0kg of ammonia and 80.0kg of oxygen are fed to a batcichine the lim which the other reactant is in excess, and the extent of the reaction (molar) and the mass proceeds to completion. First find the number of moles that corresponds to 70.0kg of ammonia (NH3). kmolNH3 Now find the number of moles that corresponds to 80.0kg of O2. kmolO2 Molar Ratio Correct. If 70.0kg of ammonia and 80.0kg of oxygen are fed to a batch reactor, determine the li which the other reactant is in excess, and the extent of the reaction (molar) and the ma: proceeds to completion. Calculate the molar ratio of O2 to NH3 fed to the reactor. O2/NH3 Using multiple attempts will impact your score. 5% score reduction after attempt 3 How many kg moles of the excess reagent are left atter the reaction proceeds to completion? kmol Hint Using multiple attempts will impact your score. 5% score reduction after attempt 3 Extent of Reaction Colculate the extent of the reaction. Hint Solution Uning multiple attempts has impacted your score. 5) score reduction after attempt 3
can i get the mass of NO in kg please 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


