Question
Can I please have help with part b. This question has multiple parts. Work all the parts to get the most points. The Romans used
Can I please have help with part b.
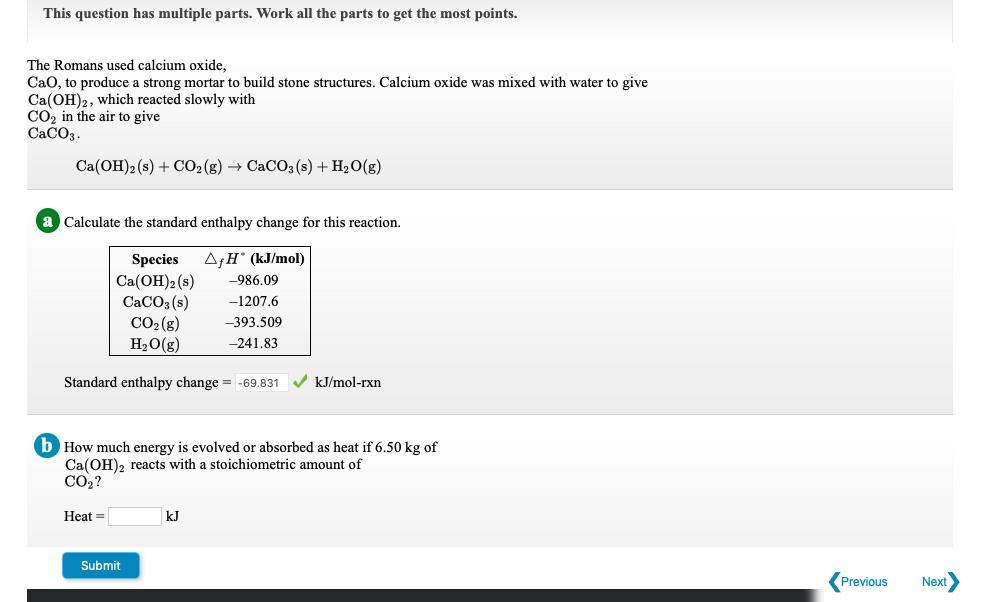
This question has multiple parts. Work all the parts to get the most points. The Romans used calcium oxide, CaO, to produce a strong mortar to build stone structures. Calcium oxide was mixed with water to give Ca(OH)2, which reacted slowly with CO in the air to give CaCO3. Ca(OH)2 (s) + CO2(g) CaCO3(s) + HO(g) a Calculate the standard enthalpy change for this reaction. AfH* (kJ/mol) -986.09 -1207.6 -393.509 -241.83 Species Ca(OH)2 (s) CaCO3(s) CO (g) HO(g) Standard enthalpy change = -69.831 b How much energy is evolved or absorbed as heat if 6.50 kg of Ca(OH)2 reacts with a stoichiometric amount of CO? Heat= Submit kJ/mol-rxn kJ Previous Next
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
5 CaOH c 0 9 AHproduct Since CaD 5 3 AH reactant Alox 69...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



