Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can i plz have help with answer all parts of these qs neatly and clearly part 1 part 2 and part 3 (i) Using the
can i plz have help with answer all parts of these qs neatly and clearly
(i) Using the following average bond dissociation energies determine the total dissociation of 1 mole of crystalline SiO2 solid. (ii) Using the following average bond dissociation energies determine the total dissociation of 1 mole of SiO2 (If it existed as individual Cin. malaruine) (iii) State which form of SiO2 takes more energy to break down into its constituent atoms and therefore which is the thermodynamically more stable. (iv) Relate the answer you gave in (iii) to the structural form and physical state that SiO2 exhibits under normal conditions. Part 2 (i) Write out the balanced chemical equations that occurred when the silicon dioxide and Mg were heated with the Bunsen burner. (ii) Give the oxidation numbers on all the atoms in each equation and therefore classify the type of inorganic reaction they both belong to. Part 3 (i) Write out the balanced chemical equations that occur when the product mixture was added to acid. (ii) State whether silanes are more or less reactive than alkanes part 1 part 2 and part 3 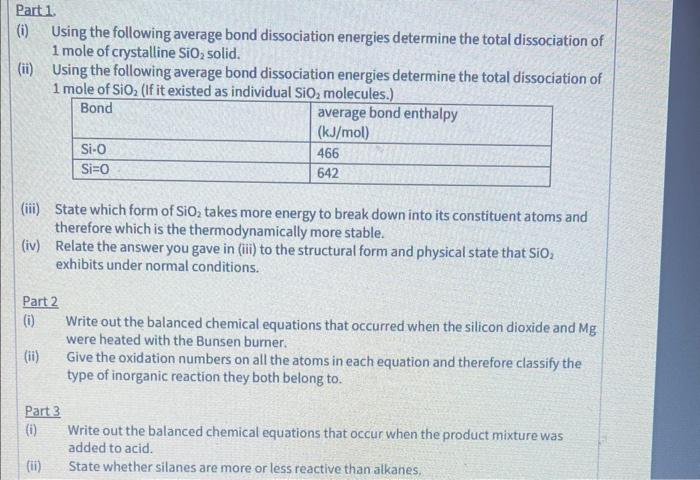

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


