Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can someone explain why these are the answers, and why does acetone and water have hydrogen bonding intermolecular force? I thought that acetone doesn't have
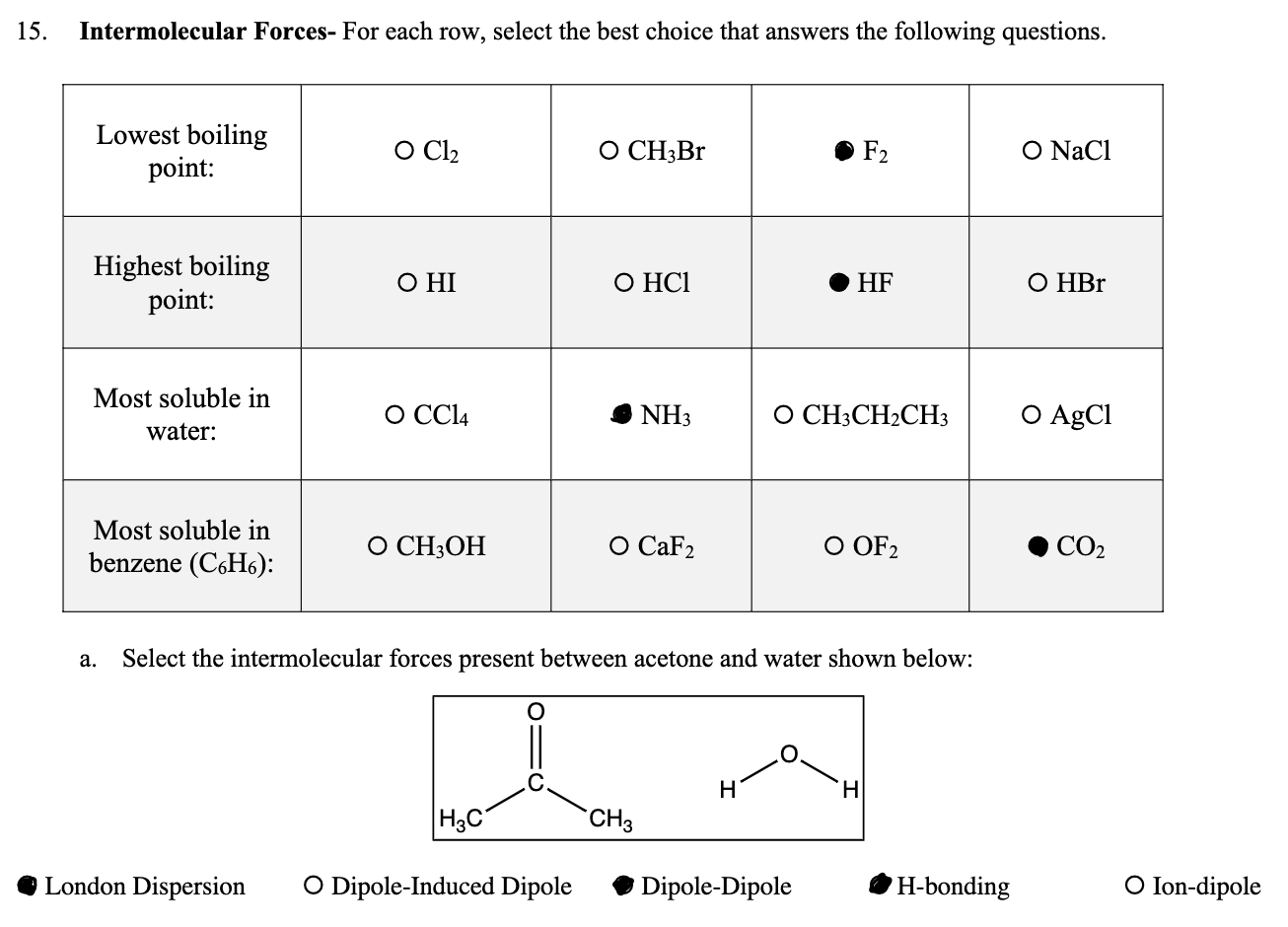 Can someone explain why these are the answers, and why does acetone and water have hydrogen bonding intermolecular force? I thought that acetone doesn't have a hydrogen bond because there isn't a Hydrogen bonded to Nitrogen, Oxygen, or Fluorine
Can someone explain why these are the answers, and why does acetone and water have hydrogen bonding intermolecular force? I thought that acetone doesn't have a hydrogen bond because there isn't a Hydrogen bonded to Nitrogen, Oxygen, or Fluorine
15. Intermolecular Forces- For each row, select the best choice that answers the following questions. Lowest boiling point: Highest boiling point: Most soluble in water: Most soluble in benzene (C6H6): O Cl O HI O CC14 O CH3OH H3C O CH3Br London Dispersion O Dipole-Induced Dipole O HC1 NH3 O CaF2 CH3 a. Select the intermolecular forces present between acetone and water shown below: H F HF O CH3CHCH3 Dipole-Dipole O OF2 H H-bonding O NaCl O HBr O AgCl CO O Ion-dipole
Step by Step Solution
★★★★★
3.52 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
C0 is polar bond and acetone is dipole OH bond is polar and H...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


