Question: Can someone help me to answer this correctly, please, and explain it For (C), the answers are below The atomic weight of thulium ( Tm
Can someone help me to answer this correctly, please, and explain it
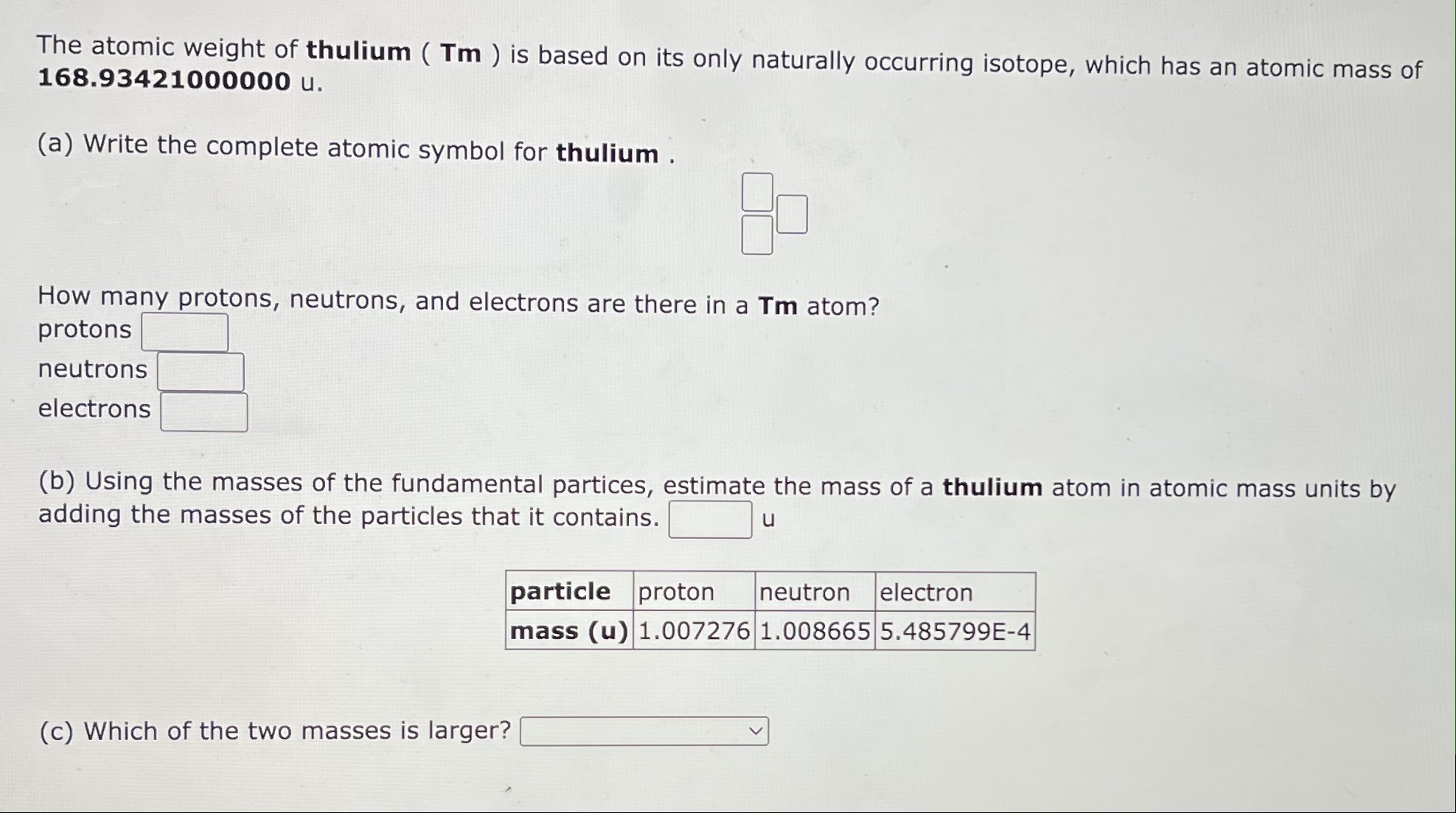 For (C), the answers are below
For (C), the answers are below

The atomic weight of thulium ( Tm ) is based on its only naturally occurring isotope, which has an atomic mass of 168.93421000000 u. (a) Write the complete atomic symbol for thulium . How many protons, neutrons, and electrons are there in a Tm atom? protons neutrons electrons (b) Using the masses of the fundamental partices, estimate the mass of a thulium atom in atomic mass units by adding the masses of the particles that it contains. (c) Which of the two masses is larger? atomic mass sum of particle masses
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


