Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you check my work please. 2. You are interested in the reaction 2AB+C. You add 10.0mMA to a solution containing an enzyme that catalyzes
Can you check my work please.
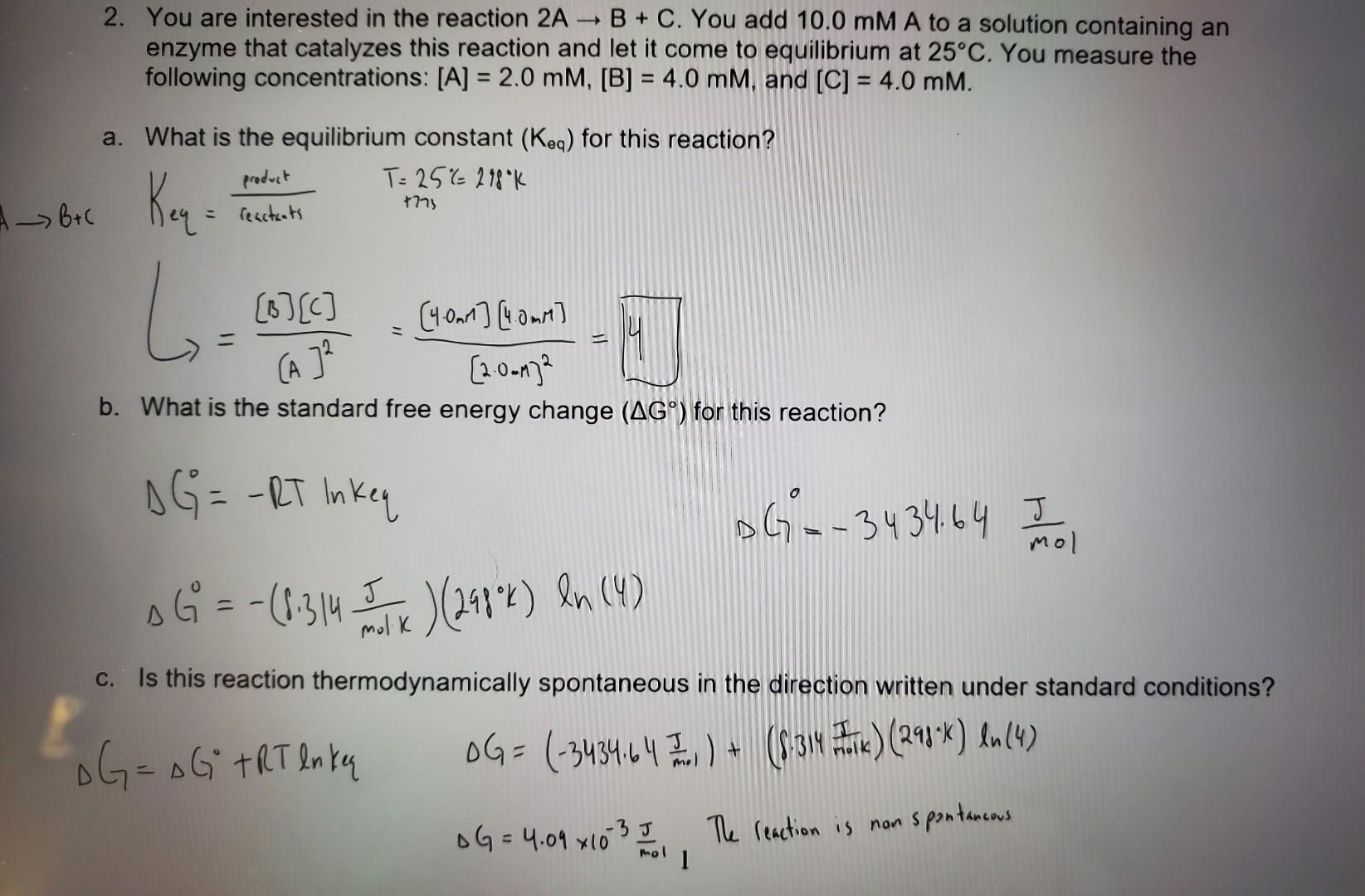

2. You are interested in the reaction 2AB+C. You add 10.0mMA to a solution containing an enzyme that catalyzes this reaction and let it come to equilibrium at 25C. You measure the following concentrations: [A]=2.0mM,[B]=4.0mM, and [C]=4.0mM. a. What is the equilibrium constant (Keq) for this reaction? Keq=reatteotspeoduttT=25%218%K+77s=(A]2[B][C]=[2.0.m]2[4.0n1][4.0mM]=4 b. What is the standard free energy change (G) for this reaction? G0=RTlnkeqDG=3434.64mol0JG0=(8.314molkJ)(298K)ln(4) c. Is this reaction thermodynamically spontaneous in the direction written under standard conditions? G=G+RTlnkeG=(3434.64molJ)+(8.314noikF)(298k)ln(4)G=4.09103molJ1Thereactionisnonspontancous d. What would be the free energy change (G) for the reaction in question 2 if you combine 1.0mM A,4.5mMB, and 4.5mMC at 25C?2AB+CG=0G+ATlnmq G=(.3140.1kJ298k)ln(20.25)=8.82105G=7452.921I e. In which direction would this reaction proceed if you now added an enzyme to catalyze this reaction? Explain
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


