Question: Can you explain this code to me in detail so I get a clear understanding? Can you also use comment code for the code as
Can you explain this code to me in detail so I get a clear understanding? Can you also use comment code for the code as well. Thank you ahead of time:-)
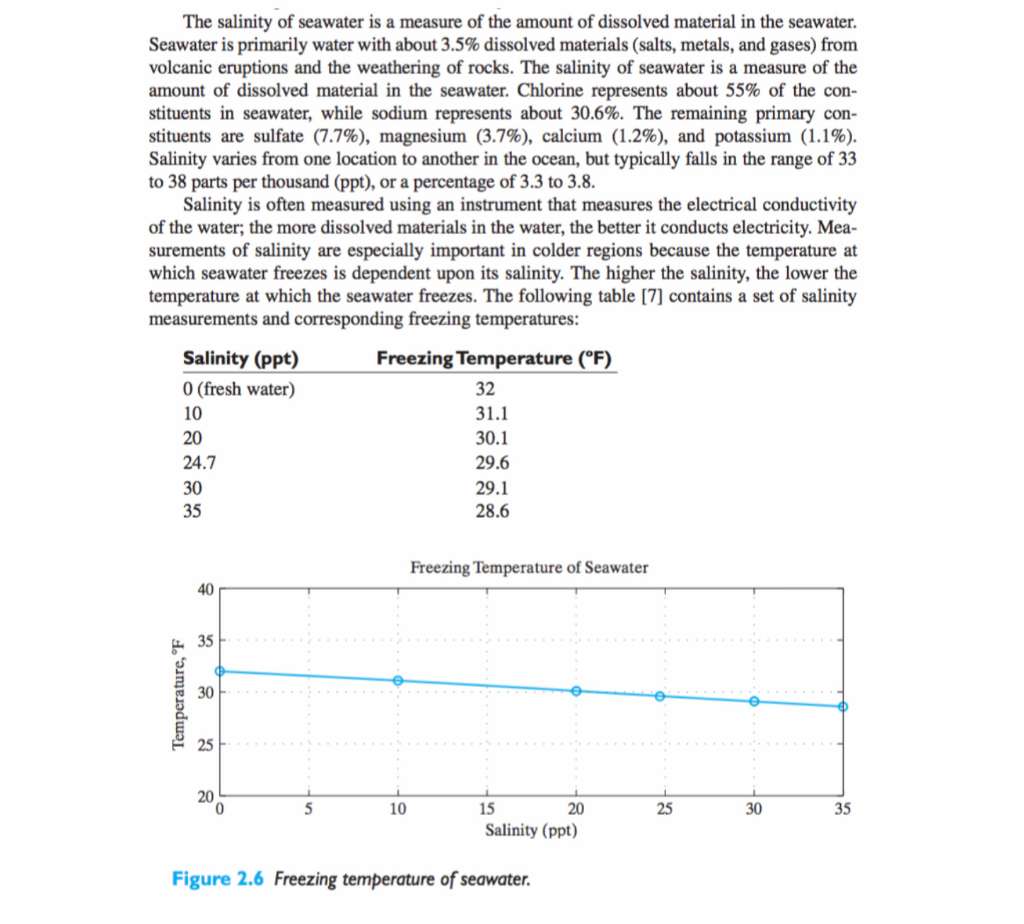

import java.util.Scanner; public class TEST { public static void main(String[] args) {
Scanner sc = new Scanner(System.in); System.out.println("Enter the lower salinity and associated freezing point temperature"); double a,b,fa,fb,c,fc; a=sc.nextDouble(); fa=sc.nextDouble(); if(a35) { System.out.println("Salinity value OUT OF RANGE"); System.out.println("Value should be in between 0 and 35"); return; } System.out.println("Enter the upper salinity and associated freezing point temperature"); c=sc.nextDouble(); fc=sc.nextDouble(); if(c35) { System.out.println("Salinity value OUT OF RANGE"); System.out.println("Value should be in between 0 and 35"); return; } System.out.println("Enter the salinity for which associated freezing point temperature is desired: "); b=sc.nextDouble(); fb=fa+((b-a)/(c-a))*(fc-fa); System.out.printf("New freezing temperature is %.1f degree F.",fb); } }
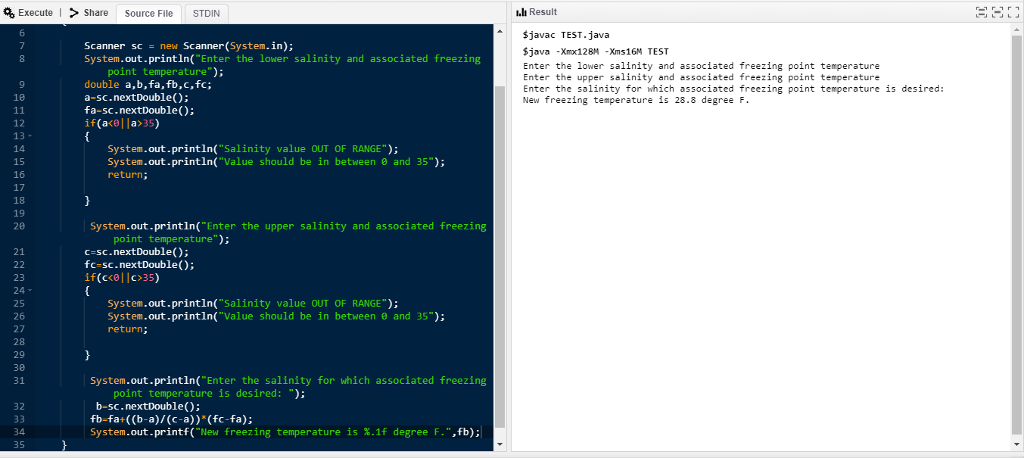
The salinity of seawater is a measure of the amount of dissolved material in the seawater. Seawater is primarily water with about 3.5% dissolved materials (salts, metals, and gases) from volcanic eruptions and the weathering of rocks. The salinity of seawater is a measure of the amount of dissolved material in the seawater. Chlorine represents stituents in seawater, while sodium stituents are sulfate (7.7%), magnesium (3.7%), calcium (1.2%), and potassium (1.1%). Salinity varies from one location to another in the ocean, but typically falls in the range of 33 to 38 parts per thousand (ppt), or a percentage of 3.3 to 3.8 about 55% of the con nts about 30.6%. The remaining primary con represe Salinity is often measured using an instrument that measures the electrical conductivity of the water, the more dissolved materials in the water, the better it conducts electricity. Mea surements of salinity are especially important in colder regions because the temperature at which seawater freezes is dependent upon its salinity. The higher the salinity, the lower the temperature at which the seawater freezes. The following table [7] contains a set of salinity measurements and corresponding freezing temperatures: Salinity (ppt) 0 (fresh water) 10 20 24.7 30 35 Freezing Temperature (F) 32 31.1 30.1 29.6 29.1 28.6 Freezing Temperature of Seawater 40 35 25 20 10 15 Salinity (ppt) 20 25 30 35 Figure 2.6 Freezing temperature of seawater Suppose that we want to determine the freezing temperature for water with a salinity meas- urement of 33 ppt. From the data, we see that this point falls between 30 and 35 ppt: a 30 29. f(a) b 33 35 286 rc) Using the linear equation formula, we can compute f(b): f(b) 29.1 3/5. (28.6- 29.1) -28.8. Problem: Use linear interpolation to compute a new freezing temperature for water with a specified salinity. The user is asked to enter a and fa), c and f(c), and b. Your program should calculate and displays f(b). If the user provides a salinity value that is below 0 or above 35, display an appropriate error. Sample Output: Enter the lower salinity and associated freezing point temperature: 30 29.1 Enter the upper salinity and associated freezing point temperature: 35 28.6 Enter the salinity for which the associated freezing point temperature is desired: New freezing temperature is 28.8 degrees F Enter the lower salinity and associated freezing point temperature: 5 30.3 SALINITY VALUE IS OUT OF RANGE VALUE SHOULD BE BETWEEN 0 AND 35 Execute>Share Source File STDIN I.lh Result $javac TEST.java Sjava -Xmox128M -Xms16N TEST Enter the lower salinity and associated freezing point temperature Enter the upper salinity and associated freezing point tempereture Enter the solinity for which associated freezing point temperature is desired: New freezing temperature is 28.8 degree F Scanner scnew Scanner(System.in); System.out.println("Enter the lower salinity and associated freezing point temperature"); double a,b,fa, fb,c,fc a-sc.nextDouble(): fa-sc.nextDouble): if(a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


