Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you find the Er using planks constant (6.626*10^-34) j/s , avogadros number 6.022*10^23 and speed of light 3*10^8m/s for each solution. can it be
can you find the Er using planks constant (6.626*10^-34) j/s , avogadros number 6.022*10^23 and speed of light 3*10^8m/s for each solution. can it be in kj/mol with the work so i can see how to do it 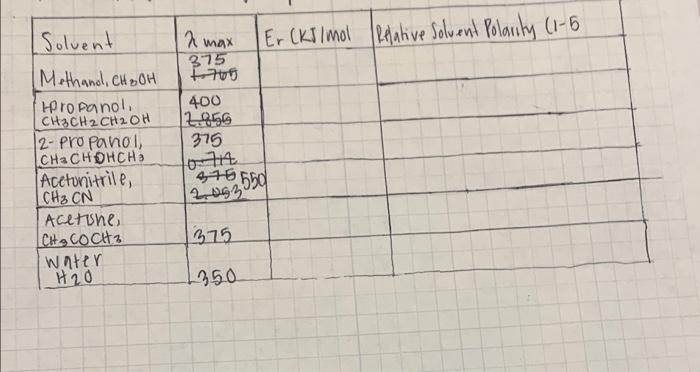
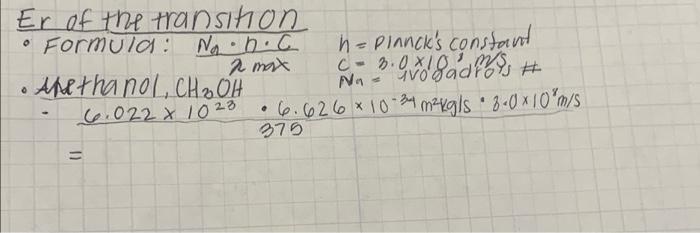
Solvent Methanol, CH BOH HPropanol, CH3CH2CH2OH 2-Propanol, CH3CHOHCHS Acetonitrile, CH CN Acetine, CHCOCH3 water H20 2 max 375 1760 Er CKJ/mol Relative Solvent Polarity (1-5) 400 2.856 375 376550 2,093 375 350 Er of the transition Formula: ND.C 2 max O Anethanol, CH OH 6.022 x 1023 = h = Planck's constant C- 3.0x8ados # Na 6.626 10-34 mkg/s 8.0 10 m/s 375 
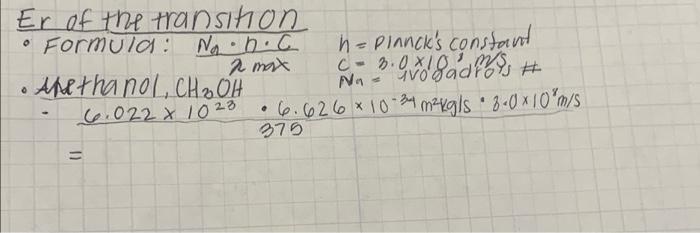
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


