Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you finish the table ? 2. Determination of Freezing Points Cyclohexane Trinit Trial 2 5.684C Tr by computer 7.153C Average Ti Trial 4 Cyclohexane
can you finish the table ? 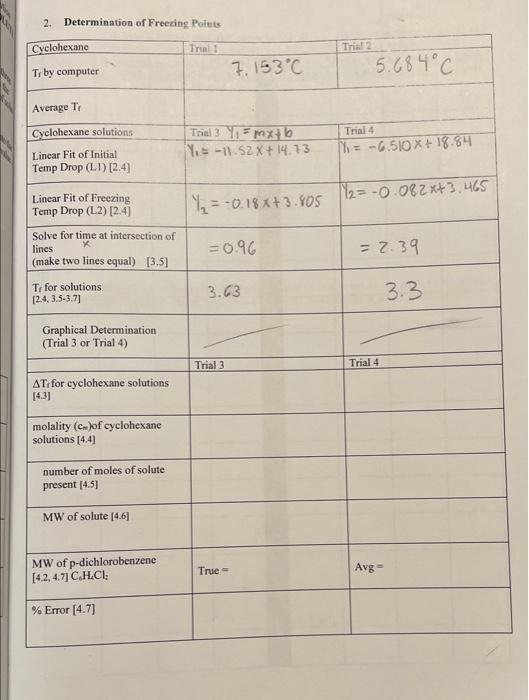

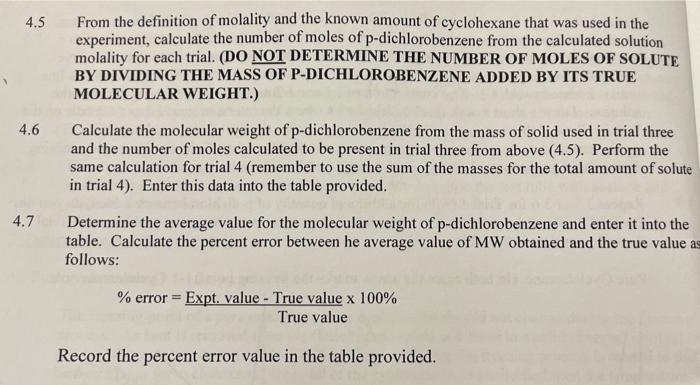
2. Determination of Freezing Points Cyclohexane Trinit Trial 2 5.684C Tr by computer 7.153C Average Ti Trial 4 Cyclohexane solutions Linear Fit of Initial Temp Drop (LD) (2.4) Trial 3 mx+b Ys -11.52 X+14.13 = -6.510 x + 18.84 =-0.18x+3.805 112= -0.082x+3.465 Linear Fit of Freezing Temp Drop (L2) [24] Solve for time at intersection of lines (make two lines equal) [3.5) Ti for solutions 12.4.3.5-3.71 = 0.96 = 2.39 3.63 3.3 Graphical Determination (Trial 3 or Trial 4) Trial 3 Trial 4 AT for cyclohexane solutions [4.31 molality (cm)of cyclohexane solutions (4.4) number of moles of solute present [4.51 MW of solute [4.61 MW of p-dichlorobenzene [4.2, 4.7] C.H.CI: True Avg - % Error (4.7) I ime 4. Calculation of Molecular Weight of p-Dichlorobenzene Note: You will have freezing point values from both the computer and by graphical analysis. Use the data from the computer method for the calculations below unless your instructor indicates otherwise. 4.1 Calculate the mass of cyclohexane used in this experiment in kg given that cyclohexane has a density of 0.779 g/mL. Enter this value in the table provided. 4.2 Calculate the true value of the molecular weight of p-dichlorobenzene given the this material has a molecular formula of C.HCl. Enter this value in the table provided. 4.3 Calculate the freezing point depression (AT) for Trial 3 and Trial 4 by subtracting the Trobtained from the average value for the freezing point of pure cyclohexane. Enter this value into the table provided. 4.4 The freezing point depression (AT) is related to the concentration of solute in molality (cm, moles of solute per kg of solvent) according the the following equation: AT,=Kr(c.) where the constant K = 20.5 C/m for cyclohexane Rearrange this equation and using the values obtained for A Tein Trial 3 and Trial 4 calculate the molality of the p- dichlorobenzene/cyclohexane solutions for each of these trials. Enter these values in the table provided. 4.5 From the definition of molality and the known amount of cyclohexane that was used in the experiment, calculate the number of moles of p-dichlorobenzene from the calculated solution molality for each trial. (DO NOT DETERMINE THE NUMBER OF MOLES OF SOLUTE BY DIVIDING THE MASS OF P-DICHLOROBENZENE ADDED BY ITS TRUE MOLECULAR WEIGHT.) 4.6 Calculate the molecular weight of p-dichlorobenzene from the mass of solid used in trial three and the number of moles calculated to be present in trial three from above (4.5). Perform the same calculation for trial 4 (remember to use the sum of the masses for the total amount of solute in trial 4). Enter this data into the table provided. 4.7 Determine the average value for the molecular weight of p-dichlorobenzene and enter it into the table. Calculate the percent error between he average value of MW obtained and the true value as follows: % error = Expt. value - True value x 100% True value Record the percent error value in the table provided 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


