Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you help me to solve this 2 questions? Determine the Ecello of a voltaic cell composed of Cu(s) in a 1.00M solution of Cu+,
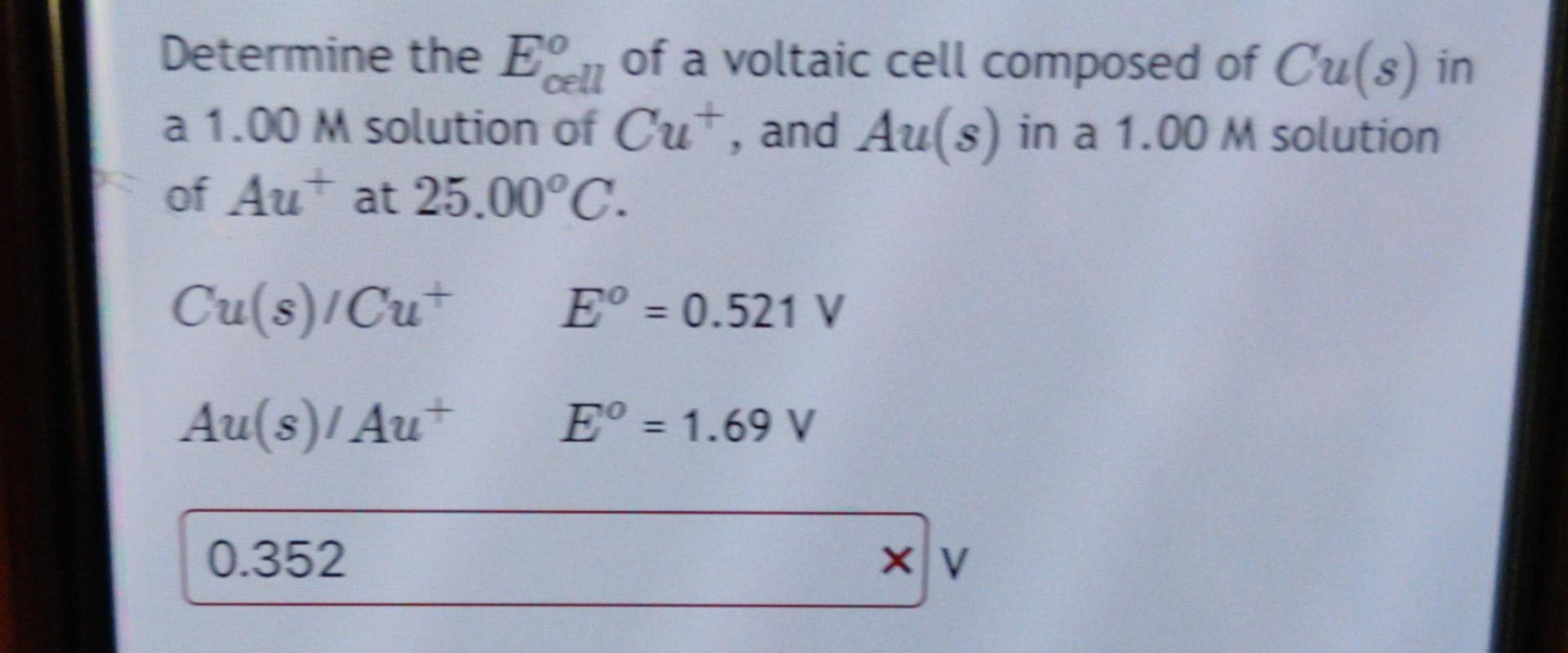

Can you help me to solve this 2 questions?
Determine the Ecello of a voltaic cell composed of Cu(s) in a 1.00M solution of Cu+, and Au(s) in a 1.00M solution of Au+at 25.00C. Cu(s)/Cu+Au(s)/Au+Eo=0.521VEo=1.69v A voltaic cell is composed of the following 2 half reactions: Cd2+(aq)+2eCd(s)Ecello=0.403Cu+(aq)+eCu(s)Ecello=0.521 Determine the non-standard state Ecell at 298K when C2+(aq)=0.267M and Cu+(aq)=0.812M. Hint: Decide which half-reaction is the reduction reaction and which one is the oxidation reaction by looking at Eo; the oxidation reaction should be reversed, and Eoxo will have the opposite signStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


