Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please do this question Crayon Length Mass Diameter 1 8.19 4.48 0.8 2 6.15 3.39 0.8 3 4.48 2.37 0.8 4 3.9 2.06
can you please do this question 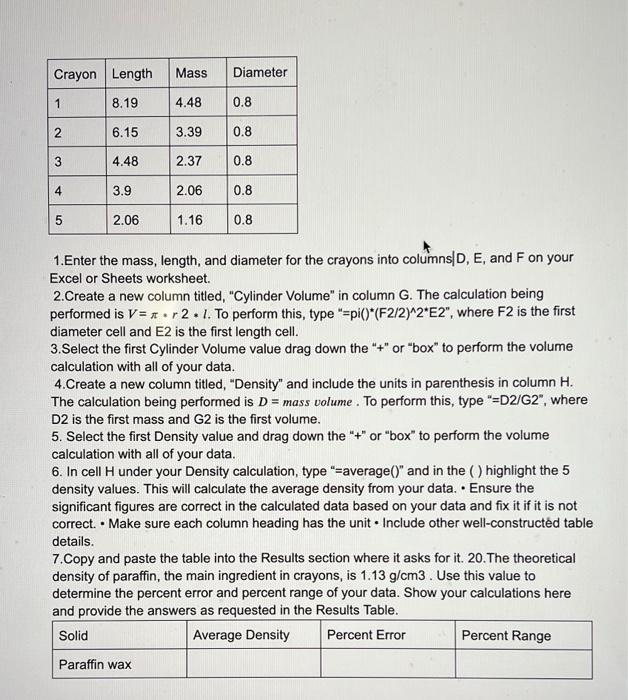
Crayon Length Mass Diameter 1 8.19 4.48 0.8 2 6.15 3.39 0.8 3 4.48 2.37 0.8 4 3.9 2.06 0.8 5 2.06 1.16 0.8 1.Enter the mass, length, and diameter for the crayons into columns D, E, and F on your Excel or Sheets worksheet. 2. Create a new column titled, "Cylinder Volume" in column G. The calculation being performed is V= xr2.1. To perform this, type "=piO*(F2/2)^2"E2", where F2 is the first diameter cell and E2 is the first length cell. 3. Select the first Cylinder Volume value drag down the "+" or "box" to perform the volume calculation with all of your data. 4.Create a new column titled, "Density" and include the units in parenthesis in column H. The calculation being performed is D = mass volume . To perform this, type "=D2/G2", where D2 is the first mass and G2 is the first volume. 5. Select the first Density value and drag down the "+" or "box" to perform the volume calculation with all of your data. 6. In cell H under your Density calculation, type "=average()" and in the highlight the 5 density values. This will calculate the average density from your data. Ensure the significant figures are correct in the calculated data based on your data and fix it if it is not correct. Make sure each column heading has the unit. Include other well-constructed table details. 7. Copy and paste the table into the Results section where it asks for it. 20. The theoretical density of paraffin, the main ingredient in crayons, is 1.13 g/cm3. Use this value to determine the percent error and percent range of your data. Show your calculations here and provide the answers as requested in the Results Table. Solid Average Density Percent Error Percent Range Paraffin wax 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


