Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you please help me with the self test. Brief illustration 21F.1 The current density The exchange current density of a Pt(s)|H2(g)|H+(aq) electrode at 298
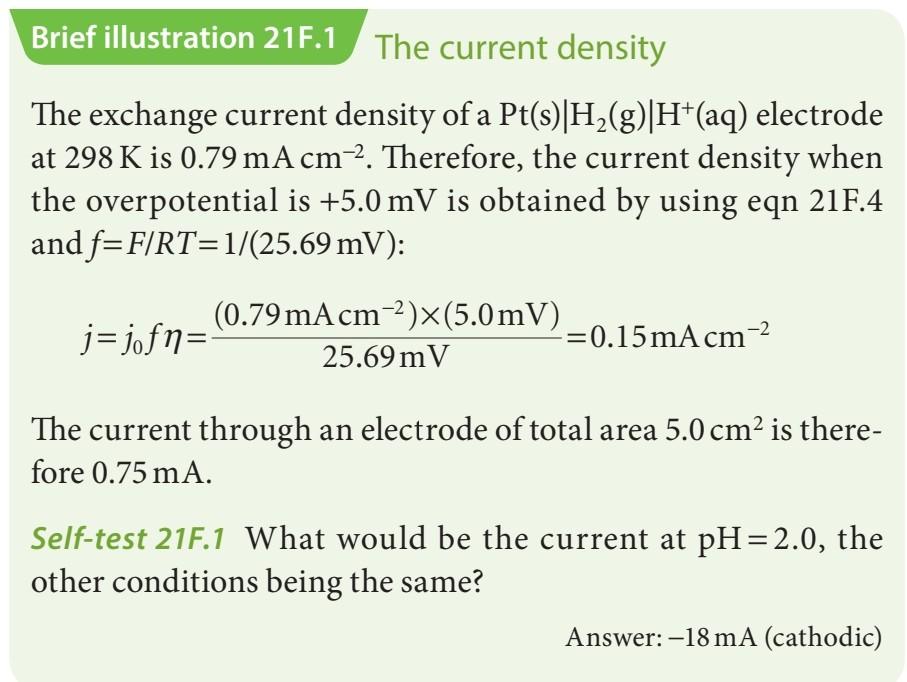
Can you please help me with the self test.
Brief illustration 21F.1 The current density The exchange current density of a Pt(s)|H2(g)|H+(aq) electrode at 298 K is 0.79 mA cm-2. Therefore, the current density when the overpotential is +5.0 mV is obtained by using eqn 21F.4 and f=F/RT=1/(25.69 mV): (0.79mAcm-2)x(5.0 mV) j=jofn= -=0.15mA cm-2 25.69 m V The current through an electrode of total area 5.0 cm2 is there- fore 0.75 mA. Self-test 217.1 What would be the current at pH=2.0, the other conditions being the same? Answer: -18 mA (cathodic)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


