Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please help me with this n-Pentane is burned with excess air in a continuous combustion chamber. Below is a skeleton flowchart Air Composition
can you please help me with this 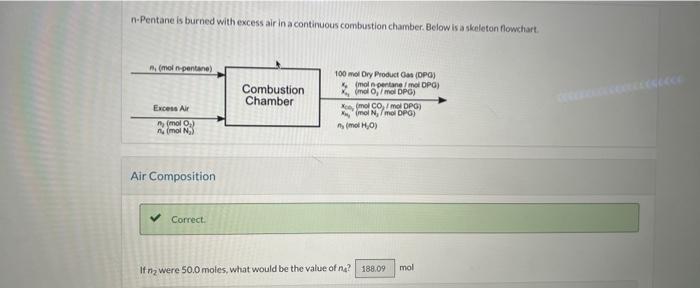
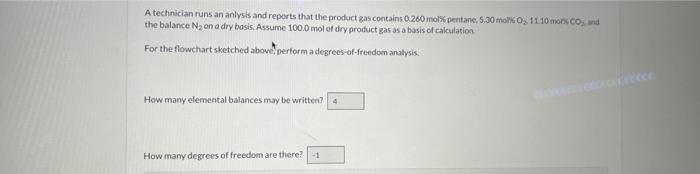
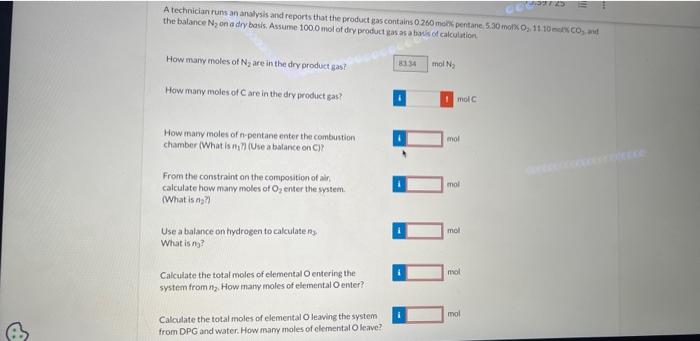


n-Pentane is burned with excess air in a continuous combustion chamber. Below is a skeleton flowchart Air Composition If n2 were 50.0 moles, what would be the value of n4 ? A technician runs an anlysis and reports that the product gas contains 0.260 molis pentane, 5.30 moki 0,11.10 monc 60 ind the balance N2 on a dry basis. Assume 100.0 mol of dry product gas as a basis of calculation. For the flowchart sketched above, perform a degrees-ot-freedom analysis. How many elemental balances may be written? How mamy degrees of freedom are there? A technician runs an analysis and reports that the product gas contains 0.260 med pentane, 5.30molO21 it 10coctCO3 - wit the balance N2 on a dry basis. Assume 1000 miol of dry product gas as a buis of caiculation. How many moles of N2 are in the dry product gas? How many moles of C are in the dry product gas? How many moles of a-pentane enter the cambustion chamber (What is 47 ) (Use a batance on C) ? From the constraint on the composition of alr? calculate how many moles of O2 enter the system. (What is ag?) Use a balance on trydrogen to calculate n3. What is nj ? Calculate the total moles of elemental O entering the: system from n2, How inany moles of elemental O enter? Caloulate the total moles of elemental Oleaving the system from DPG and water. How mamy moles of elemental O leave? A technician tuns a second analysis and reports that the product gas contains 0.60667 moly pentane, 6.8327 moki 0.9 .666 . molk CO2 and the balance N2 ona dry basis. Assume 100.0 mol of dry product gars as a bysis of calculation. How many moles of N2 ace in the dry product gas? How mary moles of C are in the dry preduct gas? How many moles of n-pentane enter the combustion chamber What is n1 ?t) (Use a bilance on C)? From the constraint on the composition of air, calculate how many moles of O2 enter the system. (What is n2 ?] Use a balance on hydrogen to calculate n2. (What is na? Calculate the total moles of elemental O entering the system fromn n2. How inany moles of elemental O enter? Calculate the total moles of elemental O leaving the system from DPG and water. How many moles of elemental 0 leave? Observe that an O balance is satisfied. What is the percentage excess of oxygen being supplied? What is the fractional conversion of n-pentane 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


